
Marine Life Society of South Australia Inc.
2003 Journal
NUMBER 13
DECEMBER 2003
Disclaimer
The opinions expressed by authors of material published in this Journal are not necessarily those of the Society.
"understanding, enjoying & caring for our oceans"
THE MARINE LIFE SOCIETY OF SOUTH AUSTRALIA Inc.
Are you interested in any aspect of marine life? Do you want to learn more about the underwater world? Are you concerned about pollution of our oceans and destruction of reefs and seagrass beds? If so MLSSA is for you.
Our motto is "--- understanding, enjoying and caring for our oceans ---". These few words summarise our aims. Members seek to understand our ocean, derive enjoyment from observations of marine life and are committed to protection of the marine environment.
Become a Society member and enjoy contact with others with similar interests. Our members include divers, marine aquarists and naturalists.
Our activities include:-
-Studying our local marine environment
-Education
-Underwater photography
-Marine aquaria
Established in 1976, MLSSA holds monthly meetings and occasional field trips. We produce various informative and educational publications including a monthly Newsletter, an Annual Journal and a beautifully illustrated Calendar showing only South Australian marine life. Our library is a source of helpful information for marine enthusiasts.
Through our affiliation with other organisations (eg Conservation Council of SA and the Scuba Divers Federation of SA) we are kept up to date with relevant issues of interest. MLSSA also has close ties with appropriate Government organisations, e.g. various museums, universities and libraries.
Everyone is welcome to attend our General Meetings which are held on the third Wednesday of every month (except December) at the Conservation Centre, 120 Wakefield Street, Adelaide, South Australia. We begin with a guest speaker. After a short break there is the general business meeting and this may be followed by a slide show if time permits. The atmosphere is friendly and informal.
We welcome new members. We have subscription levels for students, individuals, families and organisations. We invite you to complete the membership subscription form on our website at:-
http://www.mlssa.asn.auOr you may wish to write to the Society for a form, or to complete the one inside the rear cover of this Journal (or a photocopy) and send it with your payment to MLSSA.
The postal address of the Society is:-
MLSSA Inc.
120 WAKEFIELD STREET,
ADELAIDE
SA 5000
OUR LOGO
The MLSSA logo on the front page features a Leafy Seadragon which is unique to southern Australian waters. The Leafy is South Australia’s only totally protected fish. Its beauty surpasses that of any creature found in tropical waters and, once seen by divers, is amongst the most remembered of their diving experiences. The Leafy Seadragon symbolises our Society’s involvement in the marine environment.

Photograph by David Muirhead
CONTENTS
Reproductive Behaviour in the Squid Sepioteuthis australis From South Australia: Interactions on the Spawning Grounds
Fish and Cephalopod Skin Patterning
"Of Halydictyon, Jingle Shells and Brachiopoda"
Pipefish, Museums, Marine Naturalists and Fish Conservation
Air bladder surfactant in fish
Diet and feeding behaviour of the Blue-throated Wrasse
The Encounter Continues
Dragonsearch Report
Patterns of movement by Leafy Seadragons
Journal Index
EDITORIAL - Philip Hall
This edition is the largest Journal we have ever produced. I must thank MLSSA members for taking the time to contribute and to thank the other authors for their generosity in supplying articles.
As you can see from the "Contents" above, the topics are wide-ranging and I hope you find them both interesting and informative. I am sure they will give you much to think about over the Christmas Season.
Several abstracts or abridged versions of articles have been included this year. This is because the articles themselves are either too large for full inclusion, or may not yet be published in full due to copyright or other printing restrictions. Please contact the individual authors or myself to obtain the full article - where this is possible.
The Dragonsearch report in particular is an abridged version of the complete report. It makes fascinating reading and the full report (which was much too long for us to publish) should be available by the time this Journal is in your hands.
Brian Brock has included an article resulting from a favourite hobby of his - beachcombing. We are hoping that he will lead MLSSA members on some beachwalks in the near future.
During 2003 we published a series of articles in our Newsletter entitled "After the Encounter". The article in this Journal is an overview of some of the other events that followed that celebratory year.
The Journal Index is a complete listing of the major articles since the first MARIA (Marine Aquarium Research Institute of Australia) Journal way back in 1979. Please refer to the information under the heading for borrowing details.
It just remains for me to wish all readers the best for the festive season for 2003.
Reproductive Behaviour in the Squid Sepioteuthis australis From South Australia:
Interactions on the Spawning Grounds
TROY M. JANTZEN* AND JON N. HAVENHAND#
School of Biological Sciences, Flinders University,
GPO Box 2100, Adelaide, South Australia, 5001
* To whom correspondence should be addressed. E-mail: Troy.Jantzen@ bms.com
# Current address: Tjämö Marine Biological Laboratory, Göteborg University, 452 96 Strömstad, Sweden.
Reference: Biol. Bull. 204: 305-317. (June 2003)
© 2003 Marine Biological Laboratory
Abstract. Squid behavior is synonymous with distinctive body patterns, postures, and movements that constitute a complex visual communication system. These communications are particularly obvious during reproduction. They are important for sexual selection and have been identified as a potential means of species differentiation. Here we present a detailed account of copulation, mating, and egg deposition behaviors from in situ observations of the squid Sepioteuthis australis from South Australia. We identified four mating types from 85 separate mating attempts: "Male-upturned mating" (64% of mating attempts); "Sneaker mating" (33%); "Male-parallel" (2%); and "Head-to-head" (1%). Intervals between successive egg deposition behaviors were clearly bimodal, with modes at 2.5 s and 70.0 s. Ninety-three percent of egg capsules contained 3 or 4 eggs (mean = 3.54), and each egg cluster contained between 218 and 1922 egg capsules (mean = 893.9). The reproductive behavior of S. australis from South Australia was different from that described for other cephalopod species. More importantly, comparison between these results and those for other populations of S. australis suggests that behavior may differ from one population to another.
Introduction
Mate choice arises from behavioral interactions that generate selection for gender-specific traits (secondary sexual characteristics) (Ryan, 1997). Differences in reproductive success of individuals are, in turn, typically held to be caused by competition for mates (Andersson, 1994; Ryan, 1997). In systems where female choice is prevalent; sexual selection should favor conspicuous male traits that allow males to out-compete (directly or indirectly) other males (Andersson, 1994). These traits can be morphological, physical, or behavioral (Parker, 1984; Andersson, 1994; Birkhead and Parker, 1997; Ryan 1997).
In cephalopods, secondary sexual characteristics primarily consist of differences in body size, body patterns, sucker size, gonad shape or color, and sometimes photophores (Hanlon and Messenger, 1996). In squid, behavior comprises rapid body pattern changes that result from alterations in chromatic, postural, or locomotor components of behavior (Mather and Mather, 1994; Hanlon and Messenger, 1996). These behavioral patterns form a complex visual communication system. Interpreting this communication system is fundamental to understanding the processes of sexual selection in these species.
Analysis of reproductive behavior can be important when discriminating closely related species (Hanlon, 1988). Although camouflage patterns are likely to be highly conserved due to responses to common predators, reproductive communication is likely to have species-specific signals. Roper and Voss (1983) documented the range of morphological characters for species descriptions of cephalopods, and Hanlon (1988) proposed additional behavioral characters for identification. Some of the characters that Hanlon (1988) cites as being important are intraspecific agonistic behavior, mating behavior, spawning and egg care behavior, and chromatic components of body patterns. In line with these criteria, cephalopod taxa such as the squid Sepioteuthis lessoniana from Japan are now being reviewed and reclassified (Segawa et al., 1993a).
Although still regarded as a single species, geographically different populations of S. lessoniana are thought to be taxonomically different (Segawa et al., 1993a, b; Izuka et al., 1994, 1996a, b). Differences between these populations occur both at a genetic level (Izuka et al., 1994) and at a population level in differences between reproductive behavioral characteristics such as egg deposition (Segawa et al., 1993a, b; Izuka et al, 1994).
Similar uncertainty about genetic differences between geographically isolated populations of S. australis has arisen recently. Allozyme analysis of Australian and New Zealand populations of this species indicates that they are genetically distinct (Triantafillos and Adams, 2001). However, owing to the lack of comparative data on the behavior of this species in New Zealand and Australia, it is not known whether the genetic differences are expressed as behavioral differences.
The aim of this study was to identify and describe the reproductive behavior of Sepioteuthis australis from South Australia. We recently cataloged (as an ethogram) the suite of reproductive body pattern components for this species from South Australia. (Jantzen and Havenhand, 2003a). Here, we report results of underwater digital video imaging, photographs, and field notes that document the reproductive behavior of S. australis on spawning grounds over three consecutive spawning seasons. Our descriptions include previously unreported reproductive behaviors. These results are compared with previous descriptions for this, and other, squid species to identify aspects of reproductive behavior that might provide insights into secondary sexual selection in squids and the evolutionary significance of these reproductive strategies.
Materials and Methods
Mating behavior was observed during daylight hours on spawning grounds between Marino and Hallett Cove, South Australia (138° 29' E, 38° 02' S) between December 1999 and March 2002. All data were collected during the main spawning season each year (September to March). The substrate on the spawning grounds consists of patches of bare sand and rock interspersed with seagrass (Amphibolis antarctica) and brown macroalgae (Sargassum spp.) Reproductive activity of squid on spawning grounds was identified by visual observation from the surface at known locations, and by the activity of recreational and professional fishermen. Reproductive activity was observed by scuba diving and directly from the surface in less than 4.5 m of water. The presence of divers close (< 30 cm) to reproductively active individuals caused no apparent alteration in squid behavior (when compared with behavior of individuals observed from afar). Observations were therefore routinely made at a distance of less than 2 m. Still photographs of mating and spawning behavior were taken with a Nikonos V camera (Nikon Corporation, Melville, NY), and video images were recorded with a Sony TV 120 digital video camera (Sony Corporation, New York) in an Amphibico housing (Amphibico, Quebec, Canada). Video sampling followed the protocol of Martin and Bateson (1993) for focal-animal sampling, with additional ad libitum video sampling of specific behaviors. Detailed notes of reproductive activity were recorded for every observation period and compared with video and still images of behavior for the same period.
We previously identified the reproductive body patterns of Sepioteuthis australis (Jantzen and Havenhand, 2003a). The nomenclature of these patterns follows the convention of capitalizing the first letter of formally defined patterns of behaviors (Hanlon and Wolterding, 1989). Terminology applied to the physical characteristics of squid follows that described by Hanlon and Messenger (1996, fig. 2.1, p. 13). Frame-by-frame sequences of selected behaviors were obtained and analyzed using Final Cut Pro software (Apple Computer, Cupertino, CA) at a frame rate of 25 frames per second.
Durations between the completion of "Egg passing" and egg deposition, between egg deposition and "Peristaltic arm flare," and between successive observations of "Peristaltic arm flare" were analyzed using a one-way ANOVA to investigate differences between females. To meet the assumption of homogeneity of variance, data for durations between "Egg passing" and egg deposition were square-root transformed prior to analysis. These analyses were conducted to determine if these behaviors were consistent between females.
Results
Over a period of three consecutive spawning seasons, we observed more than 550 reproductively active individuals of Sepioteuthis australis in over 75 hours underwater. Observation sessions lasted as long as 120 min at a time, and the number of reproductively active squid present at any one time ranged from 2 (a single spawning pair) to more than 45 per dive. The length of time that each sex remained on the spawning grounds could not be quantified, because the size of the spawning grounds exceeded the visible range underwater. However, on all but two occasions, focal females remained in a localized area on the spawning ground throughout the observation period. In the two other instances, females swam out of view while being observed, and we do not know whether they remained on the spawning grounds. Furthermore, all observations were conducted during daylight, so we do not know if reproductive activity continued at night. Direct counts of sex ratio on each dive (and subsequent checking of these counts from the video images) showed a male-biased sex ratio between 1:1 (a single pair) and 3:1 (>4 individuals). Females were typically paired with a male, while several unpaired males swam amongst the paired individuals.
Mating
Four mating types were observed during 85 mating attempts. Mating types were classified as paired "Male-upturned mating" (PU), paired "Male-parallel mating" (PM), paired "Head-to-head mating" (PH), and "Sneaker mating" (SM). Paired mating types occurred only between paired individuals, whereas "Sneaker mating" comprised all attempts by unpaired males to mate with paired females. Of the four mating types, PU and SM were most frequently observed (64% and 33%, respectively, Fig.1), with PM and PH seen on only 2% and 1% of matings respectively (Fig. 1).
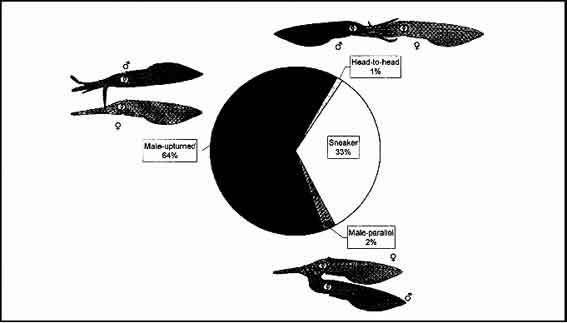
Figure 1. Percentage (of all matings) of each of the four mating, types identified in Sepioteuthis australis, with illustrations of the three mating types between paired individuals.
Paired Male-upturned mating (PU). Paired Male-upturned mating occurred most frequently (54/85 matings, Fig. 1), with a mean inter-mating interval of 7.09 min (SEM = 3.27 min, n = 11). In all cases, males swam into a position over the female prior to PU while showing "Mantle margin stripe," "Dark arm stripes," "Fin stripe," "Shaded eye," and "Rigid arms" body pattern components (Figs. 2A, 3). On six occasions, a male was seen to show up to five rapid "Lateral splayed arms" components in quick succession while above the female. This "Lateral splayed arms" behavior did not appear to evoke a response by the female.
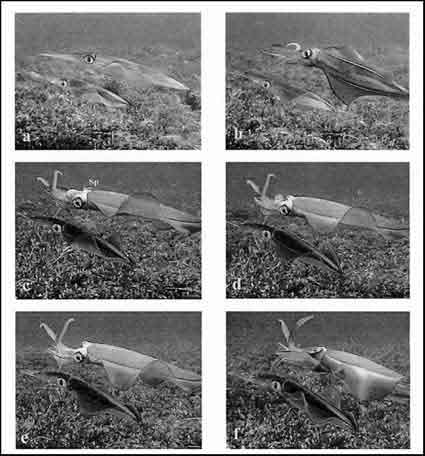
Figure 2. Six-frame sequence of "Male-upturned mating" behavior in Sepioteuthis australis. The male (top) swims into a position over the female (bottom: a). The male then rotates to the upside-down position (b) and gathers spermatophores (Sp) from the funnel with the left 4th (hectocotlyzed) arm (c). The hectocotlyzed arm then moves down the right 4th arm that is positioned in the buccal area of the female (d) and deposits spermatophores in this area (e). Copulation is complete, and the male rotates back to the normal swimming position (f). Total time elapsed = 3 s.
Once above the female, males rotated 180° around the longitudinal axis (Fig. 2B). Simultaneous with this rotation, the hectocotylized arm (left 4th arm) began moving back toward the funnel, and the right 4th arm moved toward the buccal region of the female (Fig. 2B). Once the animal was completely upside-down, spermatophores were ejected through the funnel (Fig. 2C) and gathered with a sweeping action of the hectocotylized arm across the funnel. This arm was then extended beside the right 4th arm (positioned with the tip in the female buccal region; Fig. 2D), and spermatophores were delivered into the buccal region of the female (Fig. 2E). The male then rotated back to the normal swimming position (Fig. 2F). From initial rotation of the male to completion of mating took less than 3 s.
Throughout PU, females showed "White dorsal stripe," "Golden epaulettes," and "Rigid arms" body pattern components (Figs. 2A, 3). Occasionally the posterior mantle of the female was seen to move downward 30°-40° from horizontal as the male began to rotate around to the upside-down position. PU did not occur before every deposition of an egg capsule (Fig. 3); however, this component was always observed after "Egg passing" (see below) and before egg deposition. Throughout PU, females were usually within 1 m of an egg cluster and within one body length of the substrate.
Body Pattern Component
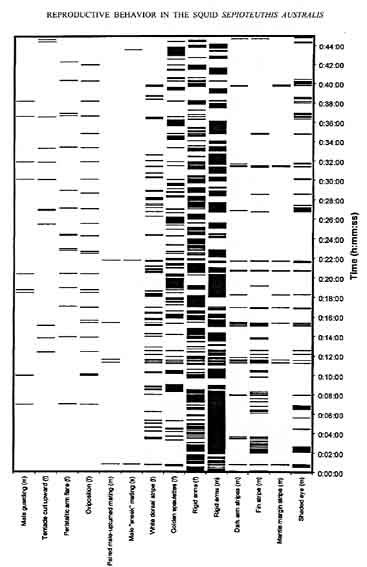
Figure 3. Signal periodicity and duration of selected components associated with egg deposition and mating from a single spawning pair of Sepioteuthis australis. Activity was recorded for a continuous focal sampling period of 45 min. Each horizontal bar represents the activity of a single body component from a single animal at any given time. Letters in parentheses indicate sex of the focal animal from which each body pattern component is recorded (m = paired male, f = paired female, s = sneaker male).
Boal and Gonzalez (1998) describe four classes of PU mating for S. lessoniana: "Pre-mating behavior" (mutual swimming of spawning pairs in a back-and-forth motion), "Flip" (whereby the male rotated around into an upside-down position), "Contact" (when the male physically contacted the female), and "Attempt" (when no contact was made with the female by the male while in the upside-down position). Only "Pre-mating behavior," "Flip," and "Contact" classes of PU had been previously described in S. australis (Jantzen and Havenhand, 2003b). We also observed the "Attempt" class of PU mating, in which mating was clearly unsuccessful (i.e., no physical contact was made with the female).
A few spermatophores (about 3-5) were seen being transferred to the female during PU. Direct counts of the number of spermatophores transferred were impossible due to the speed of PU mating (<3 s). Consequently, these numbers were estimated from analysis of digital images of two successful mating attempts and two unsuccessful mating attempts. In the two successful mating attempts, three and five spermatophores were counted. The unsuccessful mating attempts resulted in spermatophores being released into the water column, where they could be counted readily. In these unsuccessful attempts, spermatophoric reaction (the process by which sperm are ejected from the spermatophore; Mann et al., 1966) had occurred. Both times, three coagulated strands of sperm were identified.
Paired Male-parallel mating (PM). Two PM attempts were observed (2.4% of all mating attempts). On both occasions, both sexes showed the "Plain" chromatic component prior to, and throughout, PM. Our observations of PM were very similar to reports of Male-parallel mating in other squid species (e.g., Drew, 1911; McGowan, 1954; Arnold, 1962). The transfer of spermatophores to the female was not seen, and therefore we could not ascertain whether (or where) spermatophores were deposited on or in the female.
Paired Head-to-head mating (PH). This behavior was seen on only one occasion. The male swam rapidly toward the female head-on and grasped her arms and tentacles. The male remained in this position for less than 1 s before the pair separated. "Plain" was the only chromatic component seen in both sexes, and the transfer of spermatophores was not seen.
Sneaker mating (SM). On 28 occasions, an unpaired male attempted to mate with a paired female. These events were classed as SM, and occurred mostly while a paired female was attempting to deposit an egg capsule at an egg cluster, or simultaneously with the mating attempt of a paired male (Fig. 3). Four types of SM were observed: "Sneaker males" darted amongst the vegetative substrate and made contact with a female while she was at an egg cluster. Sneaker males mated in an upside-down position (consistent with the behavior of the paired male in PU mating, described above) at the egg cluster, but in the "Male-parallel" position if the paired male was "Parrying" a second unpaired male. (Note that in all cases, "Plain" was the only chromatic body pattern consistently shown by the sneaker males in these three SM types.) The fourth SM type involved sneaker males appearing to mimic a paired female. This was seen twice, and no agonistic response was shown by the paired male as the sneaker male approached the paired female. Following these mating attempts, a second unpaired male was seen attempting to mate with the sneaker males. The prominent chromatic body patterns shown by the sneaker males were "Dark mantle," followed by "Dorsal white stripe" as well as "Golden epaulettes" and "Rigid arms." These latter three body pattern components are typical of paired females throughout PU (see above).
General mating behavior. Spermatophores were generally found deposited in the buccal cavity of females; however, several females were observed with spermatophore capsules affixed to the head, arms, or dorsal mantle. The copulation attempts that led to the placement of these spermatophores were not seen; however, in one instance, a sneaker male attempted to make contact with a female on the head. The placement of spermatophores was not identified in this instance. Given that in all paired matings (and paired mating attempts), spermatophores were never seen to be placed outside the buccal cavity, it seems likely that these extra-buccal spermatophores were the result of Sneaker mating.
It was not uncommon for PU and SM to occur simultaneously (Fig. 3); on most of these occasions, females rapidly jetted away from the simultaneous mating attempts.
Egg deposition
When a female approached an egg cluster, her tentacles folded back laterally (Fig. 4a-e) as she descended toward the substrate and deposited a new egg capsule with her arms. In all cases (n = 226), attachment of egg capsules was completed in less than 2 s. Paired males regularly remained a few centimeters above the female as she attached egg capsules to a cluster ("Mate guarding"). New egg capsules appeared translucent immediately upon deposition (Fig. 4f), and the number of eggs within each newly deposited capsule was clearly visible. No more than three females were seen contributing egg capsules to each cluster. The chromatic components of females depositing egg capsules in a cluster were rarely seen, because the female was routinely obscured from view by the substrate.
Figure 4. Sequence of "Egg deposition" behavior in Sepioteuthis australis. (a) After moving into a position over the egg cluster, the female moves forward towards the egg cluster and curls both tentacles back dorsally, with the suckers on the club facing out (b). Upon nearing the cluster, both tentacles then fold back laterally (c) so that the club of each tentacle is adjacent to each eye (d). The female then attaches a new egg capsule to the existing egg cluster (e). Total time elapsed = 3 s. Following deposition, egg capsules are translucent for a short time, and individual eggs (~ 10 mm long) are clearly visible until the capsule becomes opaque (f).
The interval between deposition of successive egg capsules showed a bimodal distribution (Fig. 5). Modes in interval frequency occurred at 2.5 s and 70.0 s. This pattern was evident for each female recorded (n = 11 females). Short durations always occurred singly (i.e., were followed by at least one long duration; Fig. 3).
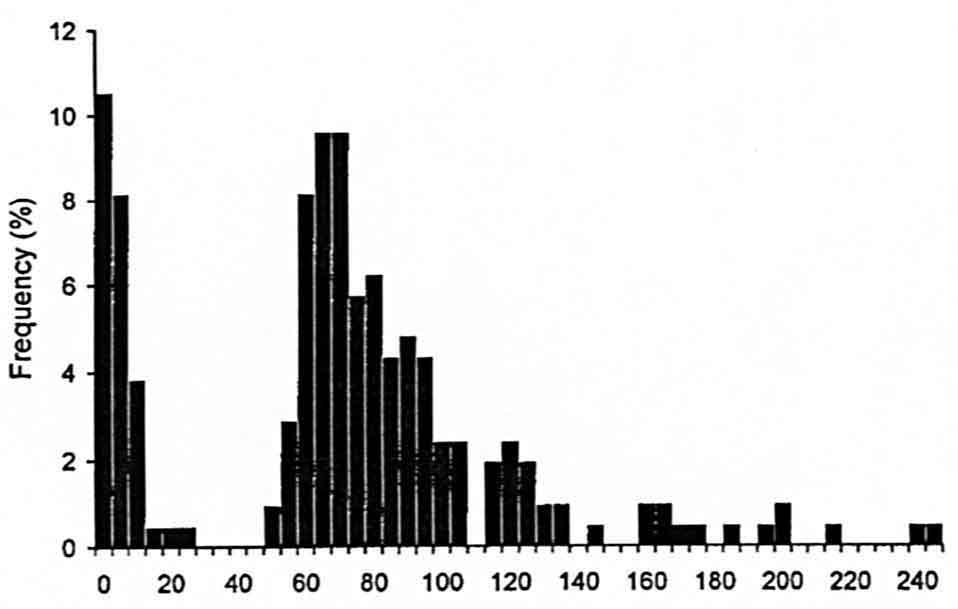
Interval Duration (seconds)
Figure 5. Frequency (%) of interval between the deposition of successive egg capsules in female Sepioteuthis australis (n = 209 durations).
Egg capsules contained 5 eggs or less with 93% of egg capsules containing 3 or 4 eggs (Fig. 6). The average number of eggs per capsule was 3.54 (SEM = 0.040, n = 300 capsules). The average number of capsules per egg cluster was 893.9 (min = 218, max = 1922, n = 9).
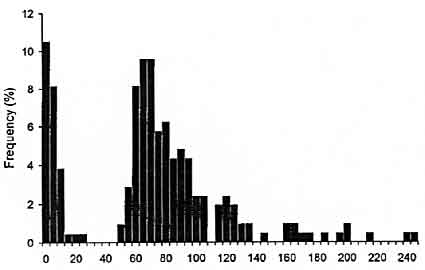
Eggs per Capsule
Figure 6. Frequency distribution (as a percentage) of egg number per capsule for Sepioteuthis australis (n = 300 capsules).
Following egg deposition, females were often seen radially flaring their arms and tentacles from the base to the tips while simultaneously pulsing a jet of water across the arms and tentacles ("Peristaltic arm flare"; Jantzen and Haven-hand, 2003a). Often, small white particles were seen rapidly expelled from the arms of the females as a result of this behavior. This "Peristaltic arm flare" occurred multiple times (commonly twice and occasionally as many as 4 times) within females. The first such event typically occurred within 20 s after the completion of egg deposition (84% of observations. Fig. 7).
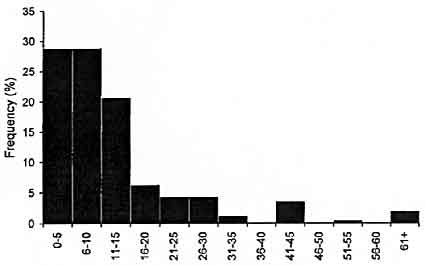
Interval Duration (seconds)
Figure 7. Frequency of observations of interval duration between conclusion of egg deposition and "Peristaltic arm flare" behavior in females of Sepioteuthis australis (n = 257 intervals).
No significant differences in the duration of the interval between the completion of egg deposition and the first observed "Peristaltic arm flare" were found among separate females (one-way ANOVA, f9,247 = 1.630, P > 0.05). This supports our observations that duration of the interval between completion of egg deposition and the first observed "Peristaltic arm flare" was consistent between females. Successive "Peristaltic arm flare" components were rapid (27% within 2 s and 58% within 10s, Fig. 8).
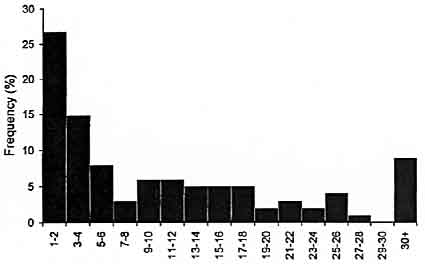
Interval Duration (seconds)
Figure 8. Frequency of observations of interval duration between successive "Peristaltic arm flare" behaviors occurring between successive egg depositions in females of Sepioteuthis australis (n = 101 intervals).
Again, no significant difference in the duration of this interval was found among 10 females (one-way ANOVA, f9,91 = 0.496, P > 0.05), suggesting that the interval between successive "Peristaltic arm flare" components is similar between females.
Egg passing
"Egg passing" denotes the process by which female squid pass eggs and associated egg capsule material from within the mantle cavity into the arms in readiness for deposition. The beginning of "Egg passing" was defined when the funnel was directed upward toward the center of the ventral arm bases. A series of peristaltic movements passed through the funnel from the mantle toward the arms for, on average, 25.8 s (n = 22, SEM = 0.6 s). Throughout "Egg passing," the ventral arm bases extended to about double their normal size (Fig. 9). Within 5 s of the completion of "Egg passing," the ventral arm bases returned to the size observed prior to "Egg passing" behavior. Neither eggs nor egg capsule material was seen throughout "Egg passing," but following this behavior the arms of the female remained in a "Rigid arms" position until the egg capsule was deposited in the cluster.
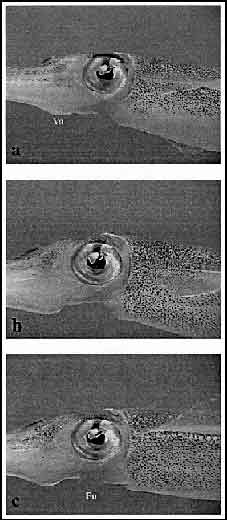
Figure 9. Three-frame sequence of "Egg passing" behavior in females of Sepioteuthis australis. Throughout "Egg passing," the ventral arm region (Va) becomes extended, and the funnel (Fu) is positioned flat against the underside of the head (immediately below the eyes; a). "Egg passing" is completed when the ventral arm region becomes substantially engorged (b), and the funnel moves back to the "normal" swimming position (c). Total time elapsed = 3 s.
The mean interval between the completion of "Egg passing" (the moment when the runnel resumed the "normal" position) and the beginning of egg deposition was 30.2 s (SEM = 4.5 n = 30 observations from 8 females). This duration did not differ significantly between females (one way ANOVA, f7,22 = 1.876, P > 0.05), which agreed with our observations that this behavioral interval was consistent between females.
Male agonistic contests
The behavior of males throughout agonistic contests was consistent with the observations of Jantzen and Havenhand (2003b). Paired males spend considerable time positioning themselves between unpaired males (these attempting to displace paired males from their mate) and the paired female ("Parrying"). "Parrying" is considered to be the very early stages of agonistic contests between rival males and was not associated with any specific chromatic body pattern components. As these contests intensified, rival males began "Fin beating" (described as "swimming fight" in Jantzen and Havenhand, 2003b). "Fin beating" occurred when both males extended their arms and tentacles and collided while swimming backwards. At this time both males showed "Dark mantle" and "Iridescent sclera" chromatic body pattern components. "Fin beating" was quite forceful between males, and the collision of mantles and fins was intense. In all fights observed (n = 67), the paired male was "victorious" such that it remained paired with the female at the completion of the fight. The unpaired was always the "loser," eventually swimming away from the pair.
Paired males also "Charged" rival males that had approached the paired female. In all observations of "Charging" (n = 7), the paired male swam rapidly at the unpaired male, striking it with the tentacles while radially flaring all arms. This is an intense agonistic encounter by a paired male and appeared similar to that described for L pealeii (King et al., 1999) and Loligo forbesi (Porteiro et al., 1990). Unpaired males always retreated following this contact.
Discussion
Mating behavior and sexual selection
About 67% of all observed mating attempts were made by paired males (Fig. 1). This indicates that paired males are able to out compete unpaired sneaker males for access to females and that paired mating has probably evolved as a more successful male mating tactic. Within paired males, three mating types were observed ("Male-upturned mating," "Head-to-head mating," and "Male-parallel mating"), with some males mating with a female in all three paired mating positions. Previously, no more than two mating positions had been reported by paired males within a single squid species (table 6.2 in Hanlon and Messenger, 1996). By far the most common paired mating type was "Male-upturned mating" (95.5% of paired mating attempts; 64% of total mating attempts), with "Head-to-head mating" (1.5% of paired, 1% of total), and "Male-parallel mating" (3% of paired, 2% of total) constituting considerably fewer mating attempts. This behavior contrasts with that of other species of squid such as Loligo plei, in which "Head-to-head mating" occurs before adults reach the spawning ground and "Male-parallel mating" occurs only when individuals have arrived at the spawning ground (Hanlon and Messenger, 1996). In Sepioteuthis australis, all three mating types were seen on the spawning ground (no data are available for possible mating behaviors prior to reaching the spawning grounds). This indicates that individual paired males of this species show considerable flexibility in mating positions. Importantly, there also appears to be flexibility in the placement of spermatohores by males. Spermatophores were most commonly deposited in the female buccal region, but they were occasionally found in the female’s mantle or on her head, arms, or dorsal mantle; they may even have been placed directly onto egg capsules as they were deposited.
In addition to outcompeting smaller males, paired males may also increase their copulation frequency as a result of mating flexibility. Different mating strategies are possibly a response that allows an individual to select the most appropriate mating strategy for the surrounding environment (Patridge and Halliday, 1984). The environmental variables influencing the mating frequency of paired male squid are likely to include not only female receptivity but also sex ratio on the spawning ground, density of reproductively active individuals, number of "sneaker" males, and susceptibility to predation while mating (although only the latter of these factors has been quantified; Smale et al., 2001). It is reasonable to assume that mating positions have evolved to provide maximum chance of successful copulation while minimizing risk to the mating pair. We have no data relating to predation risk during copulation or the potential selective benefits of different mating types; however, it is noteworthy that "Male-upturned mating" was prevalent at low and high densities (2-45+ individuals in the spawning aggregation), as well as in the presence and absence of sneaker matings.
Thirty-three percent of mating attempts were by sneaker males. Like paired males, sneaker males also showed a degree of mating flexibility in that they attempted to mate in different locations (mostly at the egg cluster but occasionally away from it), in different mating positions ("Male-upturned" and "Male-parallel"), and showing different body patterns (i.e., possible female mimicry). Sneaking behavior of unpaired males is widespread among cephalopods (Han-lon et al., 1994, 1997, 1999b; Hanlon, 1996; Hall and Hanlon, 2002; Jantzen and Havenhand, 2003b); however, despite camouflage and mimicry behaviors being used by cephalopods (Hanlon and Messenger, 1996; Hanlon et al., 1999a; Norman et al., 1999, 2001), female mimicry by sneaker males has not been reported in squid and is known only in the giant cuttlefish Sepia apama (Norman et al., 1999). Instances when sneaker males were observed to possibly mimic female behavior involved the chromatic signals "Dark mantle" followed by "Dorsal white stripe," "Golden epaulettes," and "Rigid arms" (these latter three components are characteristic signals of females copulating with paired males). The success of this apparent mimicry was evident from the observations (n = 2) that other males attempted to mate these males, and spermatophores were clearly seen being delivered to the recipient male. Given the low success rate of unpaired males in agonistic encounters with paired males (see below), it seems likely that this possible mimicry behavior has evolved so smaller males can approach paired females without instigating potentially harmful or costly agonistic contests with competing males.
It is central to sexual selection theory that differences in the reproductive success of individuals are caused by competition for mates (Andersson, 1982, 1994; Ryan, 1997). It must be remembered, however, that mating success in S. australis merely places spermatophores within the buccal region of the female—it does not necessarily result in successful fertilization. Females may mate with many males, and spermatophore longevity can be considerable (>2 weeks; Jantzen and Havenhand, unpubl. data). Consequently, female choice may play a vital role in dictating the fertilization success of sperm from different males.
The only behavior akin to direct female choice of spermatophores observed here was that in which females rejected a mating attempt by rapidly retreating ("Jetting" away). This behavior was seen only in simultaneous mating attempts of paired and sneaker males and not in (undisturbed) paired mating attempts. Consequently it seems likely that "Jetting" away was a female response to an attempted "Sneaker mating" (SM) by an unpaired male, rather than the specific rejection of a paired mating attempt. This female response to SM suggests that females are actively selecting paired males as preferred mates, which is a form of intrasexual selection (Wiley and Poston, 1996). This will add to the intense competition between males to form pair bonds with females. In systems where female choice is prevalent, sexual selection favors (among other factors) conspicuous behavioral male traits that allow males to out-compete other males (Andersson, 1982, 1994; Parker, 1984; Birkhead and Parker, 1997; Ryan 1997). It is therefore unsurprising to find intense agonistic contests between males to form pairs with females. Unlike female choice, these behavioral competitions between males for mates are a form of intersexual selection (Wiley and Poston, 1996).
In the squid S. sepioiaea, a female actively accepts or rejects spermatophores placed on her arms or head (Moynihan and Rodaniche, 1982). Although we also saw spermatophore capsules on the head, arm, and dorsal mantle of females, all spermatophores seen transferred during paired matings were deposited into the buccal region. Therefore, it is likely that those spermatophores placed on the head, arms, and dorsal mantle of females resulted from mating attempts by sneaker males.
The relationship between spermatophore placement and fertilization success is very poorly understood for cephalopods in general. In
S. australis, most SM attempts occurred while females deposited egg capsules in clusters, and any spermatophores deposited were placed on the female or directly onto the egg capsules. Paired mating attempts usually occurred prior to this egg deposition, and spermatophores were deposited primarily in the female buccal region. In squid, sperm must penetrate several millimeters of egg capsule matrix to fertilize eggs, and fertilization is thought to take place only after the egg capsule has been deposited (Arnold, 1971). Therefore, it is expected that the egg capsules from females that had been mated by two different males would routinely contain sperm from both matings, and these sperm would compete to fertilize the eggs (sensu Parker, 1970; Birkhead and Parker, 1997). Because sperm from paired matings contact egg capsules earlier (i.e., when in the arms of the female) than those from sneaker matings (i.e., during egg deposition), they have a longer exposure time to the egg capsule. Sperm contact time with eggs has been widely shown to increase fertilization success in sea urchins (e.g., Vogel et al., 1982; Levitan et al., 1991); however, comparable effects have not been tested in cephalopods. It seems likely that, as a result of longer sperm egg contact times, fertilization success of paired matings will be higher than that of sneaker matings, but the extent to which sneaker mating results in successful fertilization in S. australis has not been established.Analytical protocols to detect the paternity of S. australis embryos have recently been developed, and preliminary analysis indicates that egg clusters contain eggs sired by more than one male (L. van Camp, Flinders University; unpubl. data). Similar analyses have yet to be conducted on individual egg capsules. Multiple paternity of eggs within capsules is common in the squid Loligo pealeii and Loligo forbesi (Shaw and Boyle, 1997; Buresch et al., 2001), but details of the provenance of the males that sired the eggs (sneaker or paired) were not known. Comparable paternity investigation coupled with behavioral analysis similar to that conducted here should provide valuable information about the relative fertilization success of sneaker and paired males. Sepioteuthis australis could become a model species for this type of analysis because (1) the multiple mating strategies of males result in females regularly being copulated by more than one male throughout each spawning period; (2) scuba divers can stay close to reproductively active squid without altering their behavior; (3) the regular, short frequency of egg deposition (approximately every 70 s, Fig. 5) ensures that a large amount of data can be collected in a limited time; (4) the translucence of newly deposited egg capsules (Fig. 4f) means that each new capsule can be individually identified; and (5) the low number of eggs in each capsule (~4-5 compared to 100-300 for Loligo; Buresch et al., 2001) simplifies the analysis.
Egg deposition and population discrimination
The egg deposition frequencies of S. australis from South Australia differed from those reported for other populations of the species and for related species. Deposition of successive egg capsules in a South Australian population of S. australis occurred with clear modes at 2.5 s and at 70 s (Fig. 5), whereas a New Zealand population was reported to deposit egg capsules at roughly 5-min intervals (Larcombe and Russell, 1971). Similar, longer intervals between deposition of successive egg capsules have been reported for S. sepioidea (2-3 min; Moynihan and Rodaniche, 1982). The very short modal intervals seen here (2.5 s, Fig. 5) are insufficient for "Egg passing" to occur between capsule depositions (requiring ~ 25 s; table 1 in Jantzen and Havenhand, 2003 a). Due to the nature of our data (observational) and the egg deposition habitat (seagrass bed), we do not know whether females actually deposited two egg capsules in quick succession or whether, for some reason, they failed to deposit a capsule on the first attempt but did so soon afterwards on a second attempt. Certainly, "Egg passing" behavior did not appear to vary with egg deposition frequency. The causes of variability and plasticity in capsule deposition frequency are not fully understood (but see below); however, it is evident that even at the slower modal frequency seen here (70 s. Fig. 5), S. australis females would be capable of depositing well in excess of 50 egg capsules (~ 175 eggs) per hour.
Female body patterns during egg deposition also appeared to differ among different populations of S. australis. During egg deposition, females in South Australia folded only the tentacles back (all other arms remained in a "Rigid arms" position; Fig. 4), but females in New Zealand also folded the four lower arms down and back as they approached egg clusters (Larcombe and Russell, 1971). Larcombe and Russell (1971) also saw females pulsing a jet of water towards an egg cluster after egg deposition and interpreted this behavior as aiding in the hardening of the newly deposited capsule. "Peristaltic arm flare" was the only behavior akin to this that we observed. This behavior occurred within a few seconds of egg deposition (Fig. 6), but water was not specifically directed toward the egg clusters. Behaviors similar to "Peristaltic arm flare" have been reported in the cuttlefish Sepia latimanus (as "Puffing," Comer and Moore, 1980) to remove excess "latex-like" substance (= capsule matrix) from among the arms after egg deposition (Comer and Moore, 1980). It is highly likely that "Peristaltic arm flare" in S. australis has the same function; the behavior was observed shortly after egg deposition, and white matter was usually expelled from the arms at this time. However, it is also possible that "Peristaltic arm flare" removes spermatophores from the buccal area after fertilization of an egg capsule and before the next mating (providing another avenue for female sexual selection). The material expelled by the female in these "Peristaltic arm flare" behaviors was not analyzed, so we do not know whether it contained sperm/spermatophores or egg capsule matrix.
Investigation of the interval between successive "Peristaltic arm flare" behaviors, between egg deposition and "Peristaltic arm flare," and between "Egg passing" and egg deposition showed no significant differences among females (ANOVA, P > 0.05 in all cases, see Results). The consistency of these behaviors is surprising given the potential for variability, and it indicates that these behaviors may have evolved in response to strong selection pressures.
This study has focused on the interactions of S. australis on spawning grounds from South Australia and the implications of the observed behaviors for sexual selection. There is no comparable information on mating behaviors in S. australis populations from other regions. We have demonstrated differences in egg deposition characteristics between our data and those from geographically distinct populations in Tasmania and New Zealand. Recently, genetic and behavioral differences between populations of S. lessoniana from Japan have caused the classification of this species to be revised (Segawa et al., 1993a, b; Izuka et al., 1994, 1996a, b). Allozyme analysis of S. australis has already identified genetic divergence between geographically distinct populations (Triantafillos and Adams, 2001) but stopped short of suggesting that these are subspecies. Further genetic characterization, coupled with detailed behavioral investigations such as those reported here should provide valuable insights, not only into the variability of reproductive behaviors and the potential for differences in sexual selection between populations, but also into the importance of those differences as mechanisms of speciation.
Acknowledgments
We are grateful to J. Doube, M. Gemer, A. Hirst, D. Keuskamp, L. Kupriyanova, B. Lock, A. Mack, G. Mack, M. Naud, and V. Weisbecker for helping with underwater activities, and to C. Turner for assistance with data recording and entry. We also thank P. Fairweather and L. van Camp for valuable comments and suggestions on this manuscript. Video equipment was obtained from Sea Optics Australia Pty. Ltd. This work was supported by an Australian Postgraduate Award Scholarship (to TMJ) and a grant from the Australian Research Council (to JNH).
Literature Cited
Andersson, M. B. 1982. Sexual selection, natural selection and quality advertisement. Biol. J. Linn. Soc. 17: 375-393.
Andersson, M. B. 1994. Sexual Selection. Princeton University Press, Princeton, NJ.
Arnold, J. M. 1962. Mating behavior and social structure in Loligo pealii. Biol. Bull. 123: 53-57.
Arnold, J. M. 1971. Cephalopods. Pp. 265-311 in Experimental Embryology of Marine and Fresh-water Invertebrates, G. Reverberi, ed. North-Holland Publishing, Amsterdam.
Birkhead, T. R., and G. A. Parker. 1997. Sperm competition and mating systems. Pp. 121-145 in Behavioral Ecology: An Evolutionary Approach, 4th ed. J. R. Krebs and N. B. Davies, eds. Blackwell Science, Oxford.
Boal, J. G., and S. A. Gonzalez. 1998. Social behaviour of individual oval squids (Cephalopoda, Teuthoidea, Loliginidae, Sepioteuthis lessoniana} within a captive school. Ethology 104: 161-178.
Buresch, K. M., R. T. Hanlon, M. R. Maxwell, and S. Ring. 2001. Microsatellite DNA markers indicate a high frequency of multiple paternity within individual field-collected egg capsules of the squid Loligo pealeii. Mar. Ecol. Prog. Ser. 210: 161-165.
Corner, B. D., and H. T. Moore. 1980. Field observations on the reproductive behavior of Sepia latimanus. Micronesia 16: 235-260.
Drew, G. I. 1911. Sexual activities in the squid Loligo pealii (Les.). I. Copulation, egg laying, and fertilization. J. Morphol. 22: 327-360.
Hall, K. C., and R. T. Hanlon. 2002. Principal features of the mating system of a large spawning aggregation of the giant Australian cuttlefish Sepia apama (Mollusca: Cephalopoda). Mar. Biol. 140: 533-545.
Hanlon, R. T. 1988. Behavioral and body patterning characters useful in taxonomy and field identification of cephalopods. Malacologia 29: 247-264.
Hanlon, R. T. 1996. Evolutionary games that squids play: fighting, courting, sneaking, and mating behaviors used for sexual selection in Loligo pealei. Biol. Bull. 191: 309-310.
Hanlon, R. T., and J. B. Messenger. 1996. Cephalopod Behaviour. Cambridge University Press, Cambridge.
Hanlon, R. T., and M. R. Wolterding. 1989. Behavior, body patterning, growth and life history of Octopus briareus cultured in the laboratory. Am. Malacol. Bull. 7: 21-45.
Hanlon, R. T., M. J. Smale, and W. H. H. Sauer. 1994. An ethogram of body patterning behavior in the squid Loligo vulgaris reynaudii on spawning grounds in South Africa. Biol. Bull. 187: 363-372.
Hanlon, R. T., M. R. Maxwell, and N. Shashar. 1997. Behavioural dynamics that would lead to multiple paternity within egg capsules of the squid Loligo pealei. Biol. Bull. 193: 212-214.
Hanlon, R. T., J. W. Forsythe, and D. E. Joneschild. 1999a. Crypsis, conspicuousness, mimicry and polyphenism as antipredator defences of foraging octopuses on Indo-Pacific coral reefs, with a method of quantifying crypsis from video tapes. Biol. J. Linn. Soc. 66: 1-22.
Hanlon, R. T., M. R. Maxwell, N. Shashar, E. R. Loew, and K. L. Boyle. 1999b. An ethogram of body patterning behavior in the bio-medically and commercially valuable squid Loligo pealei off Cape Cod, Massachusetts. Biol. Bull. 197: 49-62.
Izuka, T., S. Segawa, T. Okutani, and K. Numachi. 1994. Evidence on the existence of three species in the oval squid Sepioteuthis lessoniana complex in Ishigaki Island, Okinawa, southwestern Japan, by isozyme analyses. Venus Jpn. J. Malacol. 53: 217-228.
Izuka, T., S. Segawa, and T. Okutani. 1996a. Biochemical study of the population heterogeneity and distribution of the oval squid Sepioteuthis lessoniana complex in southwestern Japan. Am. Malacol. Bull. 12:129-135.
Izuka, T., S. Segawa, and T. Okutani. 1996b. Identification of three species in oval squid, Sepioteuthis lessoniana complex by chromato-phore arrangements on the funnel. Venus Jpn. J. Malacol. 55: 139-142.
Jantzen, T. M., and J. N. Havenhand. 2003a. Reproductive behavior in the squid Sepioteuthis australis from South Australia: ethogram of reproductive body patterns. Biol. Bull. 204: 290-304.
Jantzen, T. M., and J. N. Havenhand. 2003b. Preliminary field observations of mating and spawning in the squid Sepioteuthis australis. Bull. Mar. Sci. 71: 1073-1080.
King, A. J., S. A. Adamo, and R. T. Hanlon. 1999. Contact with squid egg capsules increases agonistic behavior in male squid (Loligo pealei). Biol. Bull. 197: 256.
Larcombe, M. F., and B. C. Russell. 1971. Egg laying behaviour of the broad squid, Sepioteuthis bilineata. N. Z. J. Mar. Freshwat. Res. 5:3-11.
Levitan, D. R., M. A. Sewell, and F.-S. Chia. 1991. Kinetics of fertilization in the sea urchin Strongylocentrotus franciscanus: interaction of gamete dilution, age, and contact time. Biol. Bull. 181: 371-378.
Mann, T., A. W. Martin, and J. B. Thiersch. 1966. Spermatophores and spermatophoric reaction of the giant octopus of the north Pacific, Octopus dolfeini martini. Nature. 211: 1279- 1282.
Martin, P., and P. Bateson. 1993. Measuring Behaviour: An Introductory Guide, 2nd ed. Cambridge University Press, Cambridge.
Mather, J. A., and D. L. Mather. 1994. Skin colours and patterns of juvenile Octopus vulgaris (Mollusca, Cephalopoda) in Bermuda. Vie Milieu 44: 267-272.
McGowan, J. A. 1954. Observations on the sexual behavior and spawning of the squid, Loligo opalescens, at La Jolla, California. Calif. Fish Game 40: 47-54.
Moynihan, M., and A. F. Rodaniche. 1982. The behavior and natural history of the Caribbean reef squid Sepioteuthis sepioidea. With a consideration of social, signal, and defensive patterns for difficult and dangerous environments. Adv. Ethol. 25: 1-151.
Norman, M. D., J. Finn, and T. Tregenza. 1999. Female impersonation as an alternative reproductive strategy in giant cuttlefish. Proc. R. Soc. Lond. B 266: 1347-1349.
Norman, M. D., J. Finn, and T. Tregenza. 2001. Dynamic mimicry in an Indo-Malayan octopus. Proc. R. Soc. Lond. B 268: 1755-1758.
Parker, G. A. 1970. Sperm competition and its evolutionary consequences, in the insects. Biol. Rev. 45: 535-567.
Parker, G. A. 1984. Sperm competition and the evolution of animal mating strategies. Pp. 1-60 in Sperm Competition and the Evolution of Animal Mating Systems. R. L. Smith, ed. Academic Press, Orlando, FL.
Partridge, L., and T. Halliday. 1984. Mating patterns and mate choice. Pp. 222-250 in Behavioural Ecology: an Evolutionary Approach, 2nd ed. J. R. Krebs and N. B Davies, eds. Blackwell Scientific Publications, Oxford.
Porteiro, F. M., H. R. Martins, and R. T. Hanlon. 1990. Some observations on the behaviour of adult squids, Loligo forbesi, in captivity. J. Mar. Biol. Assoc. UK 70: 459-472.
Roper, C. F. E., and G. L. Voss. 1983. Guidelines for taxonomic descriptions ofcephalopod species. Mem. Natl. Mus. Vie. 44: 48-63.
Ryan, M. J. 1997. Sexual selection and mate choice. Pp. 179-202 in Behavioral Ecology: an Evolutionary Approach, 4th ed. J. R. Krebs and N. B, Davies, eds. Blackwell Science, Oxford.
Segawa, S., S. Hirayama, and T. Okutani. 1993a. Is Sepioteuthis lessoniana in Okinawa a single species? Pp. 513-521 in Recent Advances in Cephalopod Fisheries Biology, T. Okutani, R. K. O'Dor, and T. Kubodera, eds. Tokai University Press, Tokyo.
Segawa, S., T. Izuka, T. Tamashiro, and T. Okutani. 1993b. A note on mating and egg deposition by Sepioteuthis lessoniana in Ishigaki Island, Okinawa, Southwestern Japan. Venus Jap. J. Malacol. 52:101-108.
Shaw, P. W., and P. R. Boyle. 1997. Multiple paternity within the brood of single females of Loligo forbesi (Cephalopoda: Loliginidae), demonstrated with microsatellite DNA markers. Mar. Ecol. Prog. Ser. 160: 279-282.
Smale, M. J., W. H. H. Sauer, and M. J. Roberts. 2001. Behavioural interactions of predators and spawning chokka squid off South Africa: towards quantification. Mar. Biol. 139: 1095-1105.
Triantafillos, L., and M. Adams. 2001. Allozyme analysis reveals a complex population structure in the southern calamary, Sepioteuthis australis, from Australia and New Zealand. Mar. Ecol. Prog. Ser. 212: 193-209.
Vogel, H., G. Czihak, P. Chang, and W. Wolf. 1982. Fertilization kinetics of sea urchin eggs. Math. Biosci. 58: 189-216.
Wiley, R. H., and J. Poston. 1996. Indirect mate choice, competition for mates, and coevolution of the sexes. Evolution 50: 1371-1381.
Fish and Cephalopod Skin Patterning
By Steve Reynolds
Following my own work regarding the colours of fish (Reynolds 1980), MARIA Journal Vol.1 No. 5, I read with interest Alex Gaut’s cuttlefish article (Gaut 2000) in our 2000 Journal (No.11).
Alex gave details about the different cells that produce colour patterns in cuttles. She said that (each of) "chromatophores, iridophores and leucocytes (Fig. 1) produce colour patterns".
Leucocytes are layers of white cells. They are "under the iridophores" and "are responsible for producing white" (Gaut 2000). Iridophores (or iridocytes) are "mirror-like crystalline structures that reflect blue and green" (Gaut 2000).
Iridocyte layers have also been called iridophores, iridocystes and iridocytes (Packard & Hochberg).
Figure1 in Alex’s article seemed to be lumping leucocytes together with iridocytes/iridophores.
Layers 5 & 6 were both designated "Iridocytes" and no leucocyte layers were designated. Yet layer 6 was shown as white. The caption below Figure 1, however, explained that the "white" iridocyte/iridophore is a leucocyte layer.
Figure1 in Alex’s article was said to be from Ruppert & Barnes (1994). The diagram actually came from another publication (Nixon & Messenger 1977).
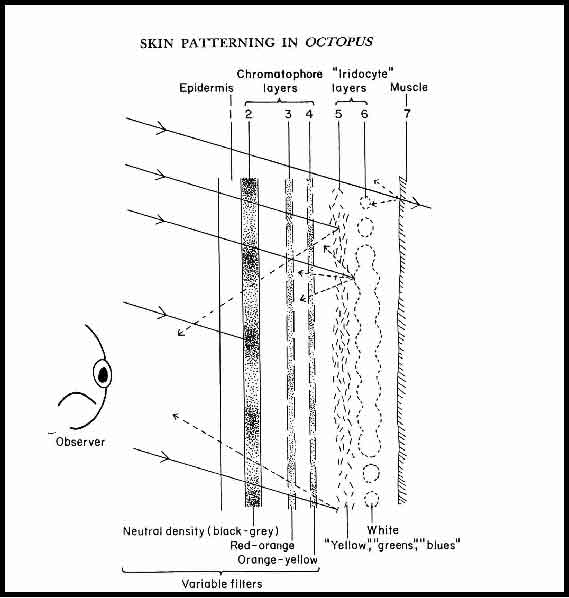
Above is Figure1 from that publication:-
The caption for Figure1 said that it is a "Schematic view of the skin to illustrate its optical properties with respect to an observer." (The ‘observer’ in Alex’s article was absent. This has been added to the diagram.)
The caption went on to say "Although generalised, the diagram may be regarded as covering the area of a single patch and its bounding grooves cut normal to the surface. Incident light passes successively through a transparent refracting layer 1 and neutral density and colour filters 2, 3 and 4 before being reflected or absorbed by layers 5, 6 and 7. Rays of light are shown as if coming from one direction only; refraction is not indicated. The variable filters are under nervous control. The neutral density filter 2 is formed of melanophores and may be tinted brown or red at the dark end of its range. Peak absorption in the colour filters 3 and 4 shifts towards the short end of the spectrum as they expand. The mirror-reflecting layer 5 reflects light of narrow bandwidth (mostly towards the short end of the spectrum) in directions that depend on the orientation of the iridocyte platelets composing it; a few orientations only are shown. The strongly reflecting white backing layer 6 scatters as well as reflects; it is composed of leucophore."
The arrows in Figure1 indicate possible absorption and reflection of light coming from the left. Layer 5 is said to be a "mirror reflecting layer" whilst layer 6 is a "strongly reflecting white backing layer (that) scatters as well as reflects".
The reason that layers 5 & 6 in Figure 1 are both described as "Iridocytes" is explained by :-
1."There are two classes of reflecting cells in the deep dermal ("iridocyte") layer of the skin: iridocytes . . . and a second class identified as leucophores" (Packard & Hochberg 1977).
2." "Iridocytes" comprise of green and blue iridocytes and white leucophores" (Packard & Hochberg 1977). The inverted commas around "Iridocytes" suggest that it is a general term for both iridocytes and leucophores.
In my own article (Reynolds 1980) I had said that leucophores were chromatophores producing white colours and that leucocytes were iridocytes containing white colours. I also said that it was iridophores that created iridescent blues, golds and silvers when they combined with chromatophores.
Fish and cephalopod skin patterning certainly is a complicated topic. When Philip Hall read my first draft for this article he told me that "Scientific terminology overlaps between countries and terms are therefore sometimes interchangeable".
Alex Gaut then told me that "the terminology differs from book to book, author to author and country to country (as it does with any science), hence making our understanding as laymen even more difficult".
My thanks to MLSSA’s Alex Gaut and Philip Hall and the SA Museum’s Thierry Laparousaz for their assistance with this article.
REFERENCES:
Reynolds 1980 – "The Colour of Fish", MARIA Journal Vol.1, No.5, September 1980.
Gaut 2000 – "The Amazing Giant Cuttle (Sepia apama), MLSSA Journal No.11, December 2000.
Ruppert & Barnes – "Invertebrate Zoology"
Nixon & Messenger 1977 – "The Biology of Cephalopods" edited by M. Nixon & JB Messenger (Available from the Barr-Smith Library at the University of Adelaide – call No. 590.6 Z8S Vol.38 ).
Packard & Hochberg 1977 – "Skin patterning in Octopus and other genera", chapter in "The Biology of Cephalopods" (see above).
Figure 1 is also shown on :-
Page 27 of MLSSA Journal No.11
Page 460 of "Invertebrate Zoology" (Figure 11-112)
Page 197 of "The Biology of Cephalopods"
FURTHER READING:
"Cephalopods – shimmering, shredders of the seas" by Mike Scotland, Dive Log Australasia, February 2001.
"How I learned to get along swimmingly with the Giant Australian Cuttlefish" by Gary Graf, GEO Magazine, Vol.9, No.1, 1987.
"The flamboyant and fascinating lifecycle of the giant cuttlefish" by Karina Hall, Southern Fisheries magazine, Vol.6, No.1, Summer 1998/99.
"Sex – Cuttle Style" by Alex Gaut, MLSSA Newsletter No.268, July 2000.
"Of Halydictyon, Jingle Shells and Brachiopoda"
By
B.J. Brock BSc, Dip Sec Ed, MScWhy go back to West Beach or the Port River, or any other site, time and again? "I’ve been there, done that!" Have you? Have you seen West Beach when westerlies are whipping sand through the dunes, squalls are sweeping in from the south, or northerlies blister along the strand? These are all part of the West Beach environment. Storm driven waves cast up gifts from the King (Neptune). Coastal vegetation and animals put up with it or shelter from it. Drift lines and beach profiles differ with every tide or gale. You may not see another human being on the beach, but you are likely to find some new "treasure" whatever the weather.
Wednesday 6/8/03 was a wild, windy day. Suitably attired, I ventured past the old wind-sculptured tamarisks at West Beach, down to the water line, and wended my way south. The drift-lines did not look very promising, but I picked up some algae and marine flowering plants. Sectioning of rhizomes of five specimens revealed that three were Heterozostera and two were Zostera. Lateral vein counts for the Heterozostera were 6/6, 4/5 and 4/4. Zostera has only one lateral vein each side of the central vein (Aston 1973, fig.120e and f).
Algae included Colpomenia, Hormosira (King Neptune lost his necklace), Sargassum, Caulerpa cactoides and several reds. I am interested in bryozoans, so collected a Sargassum plant to see what was on it (encrusting serpulids, bryozoans and colonial hydroids are common; also the occasional mollusc).
Some of our hundreds of species of extant bryozoans, look like small red algae. One small specimen picked up proved to be a Ceramium species. Another was a 3D-net-like red alga I had never seen before. There is an illustration of it in Lucas and Perrin 1947 (fig.155). See Womersley 2003 fig. 224 and pages 492-495 for a more
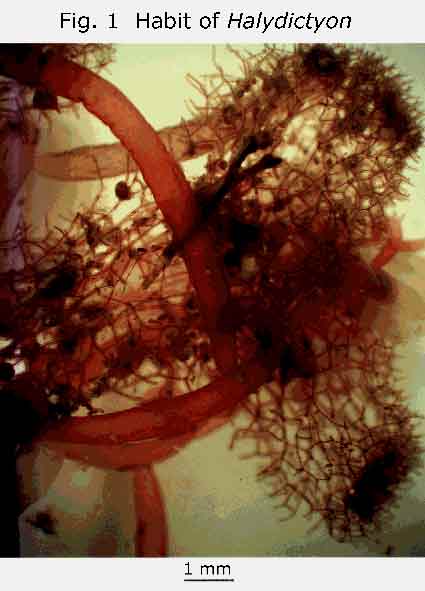
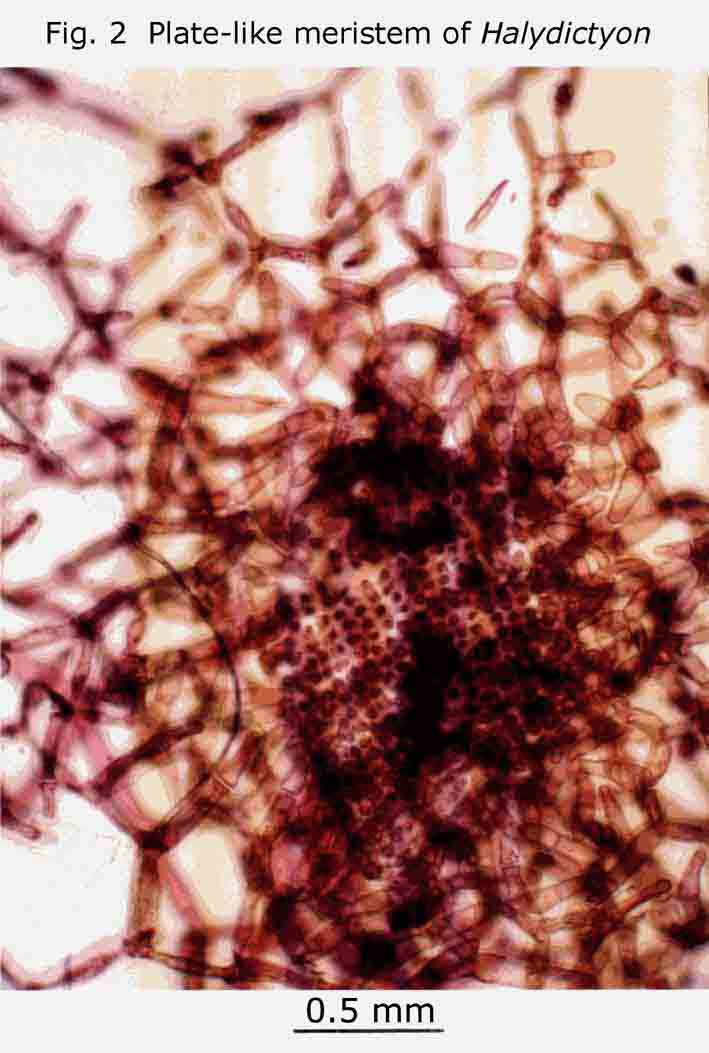
recent and detailed treatment of the genus: Halydictyon. The thallus is a sparsely branching chain of branching and anastomising monosiphonous filaments (Gr. Halysis, a chain). The little plant I found was growing on Posidonia. It was infertile. Fertile material is rare. If you find any female plants in your underwater or strand travels, and get them to the Biodiversity Centre in good condition, you will be doing the State’s phycologists a service. The habit of the plant is shown in Fig. 1, and the plate-like meristematic region in Fig. 2. My thanks to Professor Womersley for the identification and to Dr. Bob Baldock for the photographs.By a strange coincidence, I found the flat net-like green alga Microdictyon, at Coobowie many years ago. (Womersley 1984, P.217 & Fig 72). The meshes of the net are so small that the thallus just looks like a dull, yellow-green specimen of Ulva (sea lettuce) to the naked eye.
Another recent gift from the maritime gods was the Jingle Shell Anomia, which I found as drift along part of the Port River south of the Fishermens’ Market on 27/7/03. Originally called Monia ione (Cotton & Godfrey 1940; Cotton 1961 p.116), the S.A. material is now called Anomia trigonopsis Hutton (Shepherd and Thomas 1989, p.647). Having looked at the illustrations in Beu 1967, I agree that "The muscle scars and other features are typical of Anomia." (Shepherd & Thomas 1989, p.647). However, although old shells may be "pale bluish-green", as several accounts state, the most conspicuous colouration of fresher shells cast up on various beaches, including the bank of the Port River, is a lovely golden orange-yellow (Macpherson & Gabriel 1962, p.311 under Monia ione Gray 1849). The inner side of the left valve shows mother of pearl pink and green.
Some of the left valves collected on 27/7/03, had peculiar, short, columnar calcareous pillars on their outer surface. The pillars were buttressed by a gently curved "plinth" at their base. The top of each pillar consisted of several more or less parallel plates of calcium carbonate (so they could not have been solitary corals, which would exhibit radial symmetry).
The right valves of many specimens had a foramen on the dorsal margin. The pillars more or less fitted some of these. Although suggestive, I still had to find an attached Anomia shell to clarify things. I returned to the Port River on 8/8/03 and fortunately found one drift Anomia specimen still attached to its substrate (in this case, another Anomia shell). The calcified pillars were the byssal pillars of Anomia. The pillar passes through the foramen in the right valve and is attached by the byssal adductor muscle to the inside of the left valve. The arrangement of the muscle scars of this specimen is shown in fig.3.
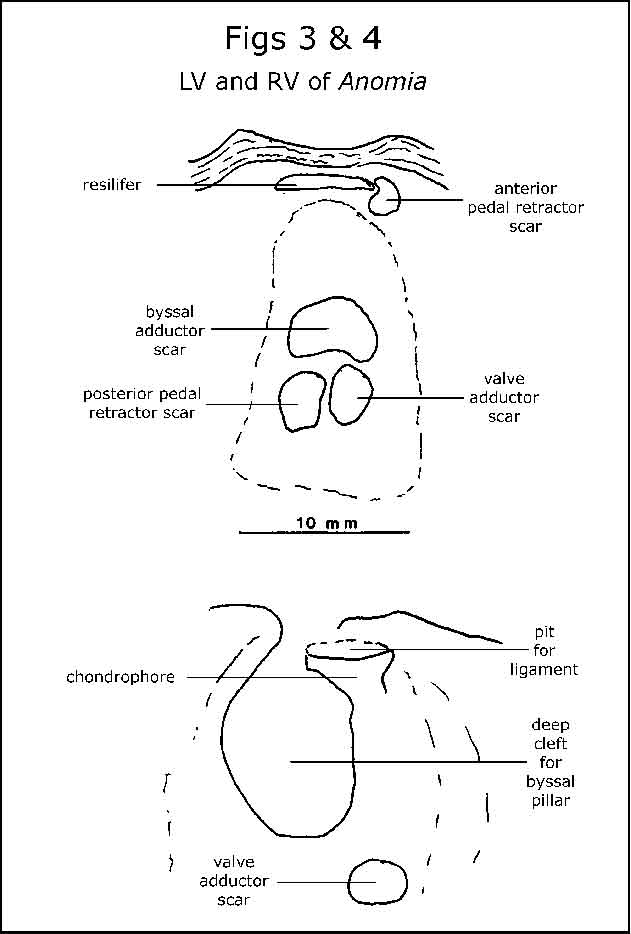
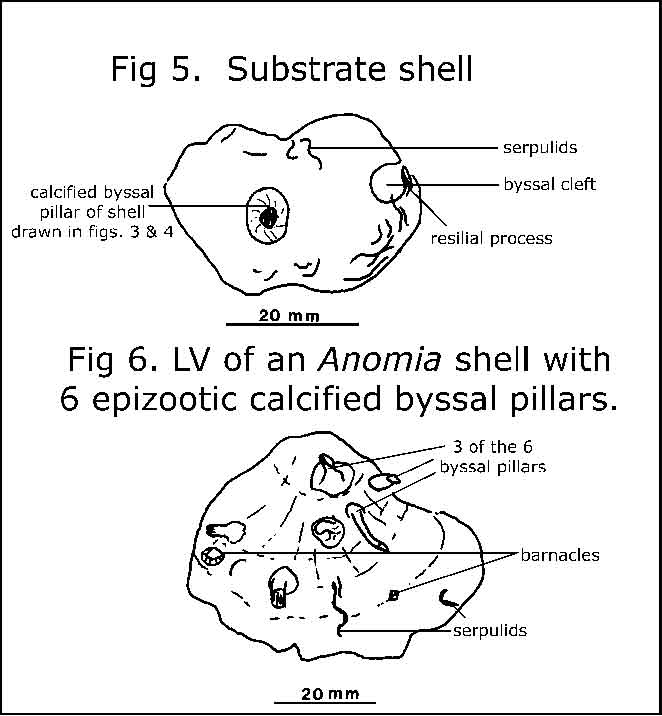
The foramen, and resilifer (for attachment of the ligament) are illustrated in fig.4; the pillar, on another Anomia shell, in fig.5. Fig.6 shows the left valve of an Anomia shell with six pillars, serpulids and barnacles on its outer surface. Why no bryozoans?
The objective of my next trip to the Port River will be to see Anomia in situ, growing and behaving in its traditional manner. How much movement occurs? What else grows on its shells? Do you find several-tiered "colonies" of living shells? What predators are there? I will no longer be able "To go with the drift(y) …..things", but "will hafta" get wet (Robert Frost, Reluctance; Roland Robinson, 1973; Brian Brock, New Year’s Resolution 2002.). I expect to find Anomia growing on ballast rocks dumped in the area.
Marine fouling biologists from various countries have recorded Jingle Shells on test plates, ships, buoys, cables and other immersed surfaces (Brock 1979; Chalmer 1979; Dunstan 1978, Russ & Wake 1975, W.H.O.I. 1952, Wood & Allen 1958). Although highly sensitive to anti-foulant toxics (W.H.O.I. 1952 p.346) Jingle Shell spat can distance themselves from the antifoulant by attaching to more resistant early colonisers. The byssal pillar is quite small and Jingle Shells are slow growers but as they become larger, they may crowd out smaller attached foulers (Chalmer 1979, p.49, for interaction with Balanus; Encyclopaedia Britannica 1961 Edn vol.16 p.999d for competition with commercial oysters). Their predilection for settling on other Anomia shells exacerbates crowding, shading and competition for suspended food.
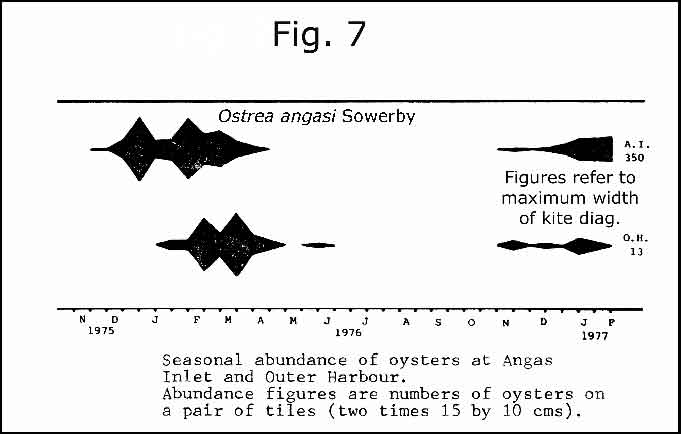
I did not have sufficient Anomia on my plates to graph seasons of settlement. However, oyster spat data is presented in fig. 7 (after Brock 1979 fig.14).
Hutchings et al (1993, p.12) recorded two species of Monia and one of Anomia in their study of infauna of maritime sediments and seagrass beds of Upper Spencer Gulf. The distinction between Monia and Anomia ought to be easy enough if muscle scars can be seen, but I do not know about the demarcation of M. ione and M. zelandica. Beau (1967) suggests that they
be synomised as M. zelandica.Fossil Anomiidae are recorded from the Miocene and Pliocene of South Australia. Beu (1967) and Burnett (1988) applied the name Pododesmus sella (Tate) to Miocene material previously known as Placunanomia sella (Tate). Beu 1967 p.242 says that Anomia trigonopsis ranges from upper Oligocene to Recent. Shepherd & Thomas 1989 p.647, use this binomial for extant material as well.
Ludbrook, 1955, applied Anomia tatei Chapman & Singleton to her chosen Pliocene hypotype and 34 other specimens from the Abbatoirs Bore. Tate, 1890, used the name Placunanomia ione Gray. The Pliocene material has also been called Monia ione Gray (Ludbrook as Woods, 1931) and Monia tatei Chapman & Singleton (Cotton, 1947). As can be seen from Ludbrook 1955 Plate 4 fig.11 (her "hypotype"), the muscle scar pattern is typical of Anomia (3 scars) rather than Monia (2 scars). See Beu 1967, for the scar patterns of the two genera. I suspect that Ludbrook would have been happy to apply Beu’s Anomia trigonopsis Hutton, to the Pliocene material later (Ludbrook 1984 p.165). It would be interesting to find out whether any of her Abattoirs Bore specimens, showed evidence of attachment by other Anomia shells. I have not seen the Pliocene fossils or all of the Anomia literature. Their nomenclature is an intriguing maze.
Jingle Shells ought to be satisfactory aquarium subjects. How about trying Lamp Shells as well. Cotton 1964 p.14, records the Brachiopod Magellania flavescens attached to rocks South of the breakwater at Outer Harbour. I have found the occasional specimen on nearby beaches. Lamp Shells attach to the substrate by a stalk passing through a foramen in the ventral valve (see Hyman 1959, figs.184J and 190A for Magellania; fig.189F is of a Brachiopod dredged from 2253 metres in the Indian Ocean. It has a long stalk or "pedicle").
If collecting Lamp Shells or Jingle Shells for aquaria, I suspect that the substrate to which they are attached would also have to be collected, to avoid killing the animal. One approach might be to place suitable substrate near existing colonies and wait for settlement to occur.
I have only found about five drift Lamp Shells in a long beach-combing career (one at Aldinga; two at Outer Harbour; two at Port Hughes). If that is an indication of their rarity, perhaps they ought not be collected live for aquaria at all.
REFERENCES
Aston H.I. (1973) Aquatic Plants of Australia (Melbourne University Press.)
Beu A.G. (1967) Notes on Australasian Anomiidae (Mollusca, Bivalvia) Trans. R. Soc. New Zealand. Zoology 9(18), 225-243.
Brock B.J. (Unpub.) Biology of Bryozoa Involved in Fouling at Outer Harbour & Angas Inlet. M.Sc. thesis submitted 17.10.79 University of Adelaide.
Brock B.J. (Unpub.) New Year’s Resolution 2002. Poem.
Burnett J. (1988) What fossil is that? South Australian Science Teachers Association Journal No. 88/1. Pp17-32. Pododesmus sella (Tate) & five spp. of Brachiopoda are included in fourteen pages of illustrations.
Chalmer P.N. (1979) Some Mechanisms of Succession in a Marine Fouling Community. Ph.D
thesis University of Western Australia.Cotton B.C. (1947) Some Tertiary Mollusca from the Adelaidean Stage (Pliocene) of South Australia. Rec. S.A. Museum 8(4)653-670. On p654 "Monia tatei Chapman & Singleton. Recorded as Placunanomia ione Gray".
Cotton B.C. (1961) South Australian Mollusca Pelecypoda. (Govt Printer : Adelaide.)
Cotton B.C. (1964) Beachcombing at the Outer Harbour South Australia. (South Australian Museum).
Cotton B.C. & Godfrey F.K. (1940) Sea shore shells of South Australia. The South Australian Naturalist 20,(4), pp49-60. Monia ione p.55.
Dunstan I.C. (1978) Marine Fouling at HMAS Stirling W.A. Oct.1976-April 1978. (Dept of Defence Materials Research Laboratories report MRL-R-731.)
Encyclopaedia Britannica (1961) Jingle shells (Anomia spp) a problem to oyster growers. Vol. 16 p.999d.
Frost R. (1951) Reluctance. In R. Frost Complete Poems of Robert Frost, pp 49 & 50.
Hutchings P.A. et al (1993). Infauna of marine sediments and seagrass beds of Upper Spencer Gulf near Port Pirie, South Australia. Trans. R. Soc. S. Aust. 117(1), 1-15.
Hyman, L. H. (1959) The Invertebrates Smaller Coelomate Groups......Volume V. (McGraw-Hill Book Company.)
Lucas A.H.S. & Perrin F. (1947) The Seaweeds of South Australia Part 11 The Red Seaweeds. (Govt Printer : Adelaide.)
Ludbrook N.H. (1955) The Molluscan fauna of the Pliocene strata underlying the Adelaide Plains. Part 11 - Pelecypoda. Trans. R. Soc. S. Aust. 78 pp 18-87.
Ludbrook N.H. (1984) Quaternary Molluscs of South Australia. (Dept of Mines & Energy Handbook No. 9.)
Macpherson J. H. & Gabriel C.J. (1962) Marine Molluscs of Victoria. (Melbourne University Press & the National Museum of Victoria.)
Robinson R. (1973) The Drift of Things. (Macmillan Australia Pty. Ltd.)
Russ G.R. & Wake L.V. (1975) A Manual of the Principal Australian Marine Fouling Organisms. (Department of Defence Materials Research Laboratories Maribyrnong Victoria. Report 644.)
Shepherd S.A. & Thomas I.M. (1989) Marine Invertebrates of Southern Australia. Part 11. (Govt Printer : Adelaide.)
Tate R. (1890) On the discovery of marine deposits of Pliocene Age in Australia. Trans. R. Soc. S. Aust., 13(2) pp 172-180. On p175 Placunanomia ione Gray. Miocene. Pliocene. Recent.
Womersley H.B.S. (1984) The Marine Benthic Flora of Southern Australia Part 1. (Govt Printer : Adelaide.).
Womersley H.B.S. (2003) The Marine Benthic Flora of Southern Australia Part 111D. (A.B.R.S. Canberra & State Herbarium of South Australia, Adelaide.)
Wood E.J.F. & Allen F.E. (1958) Common Marine Fouling Organisms of Australian Waters. (Dept of the Navy. Melbourne.) P16 Anomia descripta Iredale. (Plate 24 also).
Woods N.H. (1931) Pelecypoda from the Abbatoirs Bore including 12 new species. Trans. R. Soc. S. Aust. 55 pp145-151.
Woods Hole Oceanographic Institution (1952) Marine Fouling and its Prevention. (United States Naval Institute. Annapolis, Maryland.)
By
Dr Robert BrowneRobert lived in South Australia for forty years before beginning a career in science. He has a wide interest in conservation biology from reproductive physiology and gene banking of threatened species to broad scale fauna assessments and ecological studies.
One of those idyllic days of the autumn of 2003, still, warm and clear, I was on the rocks south of Hallett Cove looking at rock pool fish. My friend who was snorkeling over the seagrass beds beached himself and held up a hand net. He showed me a tiny green fish about 2.5 cm long. What do you think it is? As this fish was not listed in our fish books, into the South Australian Museum it went. Two days later we were told it was a grass clingfish, a new record for the State. The finding a novel species so close to Adelaide and so close inshore begged the question, "How many other inshore marine fish species were unrecorded?".
Although much of my childhood during the 60s was spent exploring the coast south of Brighton, my main interaction with fish mainly consisted of harvesting them with rod or spear. However, I was also a naturalist and attempted to identify, and find out the natural history of, the various fish I encountered. Due to a strong history of natural sciences in South Australia up to the 1980s, the State was endowed with the most comprehensive guides to marine fish in Australia. The accuracy of these guides was confirmed by beautifully preserved museum specimens (Figure1), the legacy of pioneering marine naturalists who named most fish species mentioned in this essay.

Figure 1
These Short-snouted Seahorses were collected by Sir Joseph Verco in 1920. Sir Joseph Verco personally financed many marine expeditions and greatly contributed to the knowledge of marine biodiversity in South Australia.
However, even with the available literature, the identification of marine fish, except for large common species, was a difficult and frustrating experience. Many just didn’t match. The long hours attempting the identification of a novel fish usually ended with "Well it could be that one but then again! …" My growing amateur interest and desire to contribute to the knowledge and conservation of South Australian fish was severely curtailed.
In 1923 Mr. Edgar R. Waite published a catalogue of the fishes of South Australia. "There is a lack of inexpensive but accurate books dealing with the plants and animals of South Australia, the absence of such has been a real handicap to young Australia, and so to the progress of Australian science." He considered the catalogue accurate, "As far as the more familiar fishes are concerned, it may be accepted as reliable, for the gaps in our knowledge relate mainly to small, rare and obscure forms." He used as an example the seahorses and pipefish (Family: Syngnathids) "At least six were found to be incorrectly determined, and the examination of the single group revealed one new genus and five new species." Waite’s catalogue listed ten pipefish. We now consider that there are at least thirty-four Syngnathids in South Australia and there are probably many more.
The Syngnathids of southern Australia are important from a global perspective. Of approximately 210 species in 52 genera worldwide listed in Dawson 1985, almost half in 38 genera exist in Australia. Of these 38 genera, 37% are regarded as endemic, with 25% of the world’s species considered endemic to Australia. Of the currently recognized 330 species, about 36% occur in Australian waters. Of these, many exist in monotypic genera and are of particular significance in respect to ecology, biogeography and phylogeny, thus their conservation is particularly important. Of the pipefish, ten of the world’s 14 genera are endemic to Australia; from Bermagui, NSW, there are 23 species, of which 25% are Australian endemics and 17% regional endemics; and from Shark Bay, WA, to Robe, SA, there are 38 species, of which 41% are Australian endemics and 29% regional endemics. Similar endemicity also applies to many other southern Australian inshore demersal (bottom dwelling) fish.
This endemicity occurs because Australia was long isolated from other continents and the coast of southern Australia is the longest east-west temperate coastline in the world, and therefore a unique marine biogeographical region. Further, South Australia’s coastlines unique northward curve in the Great Australian Bight supplemented by the Leeuwin Current from Western Australia, and the northern end aspect of Spencer Gulf and, to a lesser extent, St Vincent Gulf, have provided warm isolated habitats enabling the evolution and survival of relict sub-tropical species. South Australia also has a wide variety of marine habitats for fish, including many enclosed bays separated by deeper, high energy coastlines, and within these bays are micro-habitats, provided by the greatest variety of macro-algae in the world.
For instance, Barry Hutchins from the Western Australian Museum says, in respect to clingfish, "We have been actively surveying reef and sea grass fauna. Countless new species have been discovered. Only two species of clingfish were known from seagrass habitat. We now have over 20 species. It is not known how many species are shared with South Australia, but I would suspect that the number would be considerable". The gobies, weedfish, and snake-blennies are other inshore fish where many new species can be expected. Novel inshore fish species are being frequently discovered using hand nets, or lines with small hooks, even at places near Adelaide, like West Lakes and the Port River.
A comprehensive knowledge of inshore fish is particularly important for their conservation because a large number of fish species are concentrated within the first five metres of depth in bays and estuaries. These rich inshore areas are subject to increasing recreational use and development. The obvious lack of information on inshore demersal fish limits their conservation and consequently the conservation of global marine life.
It is now quite clear that the conservation of broad habitat types, even though essential to the conservation of many species, offers little protection to some. Therefore a sound knowledge of the diversity and distribution of individual species is essential to their conservation. The demise of many mammals in uncleared forest and brushland was due to exotic predators, competitors or diseases. Further, all frog species that have reached extinction in Australia have done so as a result of introduced disease, not of broad habitat change. In fact many of these extinct frogs inhabited regions of pristine rainforest in national parks. It is relevant that the extinction of many of these species was not recorded until many years after their demise, and only after overseas extinctions were recorded, because there was no systematic monitoring of frog populations. In respect to the monitoring of populations, the situation with inshore demersal fish today is similar and probably worse than that which occurred with the extinct frogs.
Introductions of disease, exotic predators and competitors are occurring at an accelerating rate in the marine environment. Hopefully, quarantine will slow the introduction rate of these pests. Nevertheless, many of these pests are increasing their range. In the Derwent estuary, Tasmania, the Spotted Handfish, Brachionichthys hirsutus, has been endangered by the introduction of the Northern Pacific seastar, Asterias amurensis. Similarly, the European fan worm (sabellid) which has occupied large areas of Port Phillip Bay in Victoria, and which is now found in South Australia, may endanger inshore demersal fish. As the European fan worm feeds on zooplankton and replaces shelter, its effect on plankton feeders such as pipefish may be particularly detrimental. There have also been records of exotic crabs including established populations of the European Shore Crab - crabs are a major predator of pipefish- in South Australian waters. Invasive Caulerpa taxifolia also poses a clear threat to other marine species. Mass die-offs of the Common seadragon, Phyllopteryx taeniolatus, were noted by Dragon Search and these corresponded to novel viral epidemics in pilchards. Such events could lead to population loss in other Syngnathids such as pipefish.
This suggests that in the future many marine species will decline or reach extinction in the wild. The pressures on the marine environment are unrelenting. For example, worldwide 90% of large predators have been removed by fishing, thus producing imbalance in the food chain, and the oceans continue to be polluted, by both chemical agents and by the physical agent of greenhouse warming. In the shallow northern end of Spencer Gulf the effects of climate change are exacerbated by warming from power stations. This area holds many unique species of marine life and is poorly known and monitored.
There is no reason to assume that many of the current generation will not continue to recklessly and ignorantly destroy the ocean’s ecology. However, there is also no reason to assume that many future generations will not prefer the sustainable use of natural resources. Imagine if 40 years ago society had said "Why conserve whales, they’re almost extinct, what’s the point?", or had said "Why bother setting up national parks?". Thus, although we accept a marine conservation crisis, we also realize that many species can be saved in the wild, and others can be preserved for reintroduction in the future.
Fortunately, for species which will become extinct in the wild, programs are being developed for their preservation. Some of these programs are incidental, such as the aquaculture of seahorses, and encourage economic development. Other programs for the conservation of non-commercial endangered species consist of population enhancement through captive breeding, with genetic diversity maintained through genetic resource banks, offering the best cost to benefit solutions. Captive breeding is currently being used for the preservation of the Tasmanian Spotted handfish. Stocks, and genetic diversity, of many commercial and non-commercial fish throughout the world are already maintained by these methods. For fish and amphibians (frogs) technologies to enable the indefinite preservation of endangered species through cryopreservation are being developed. As other technologies develop, the introduction of genes conferring immunity to exotic diseases will enable the re-establishment of populations lost through this caue. Similarly the future eradication of marine pests or genetic technologies may allow the reintroduction of endangered marine species conserved through captive breeding and genetic resource banks. However, no conservation program can be successful without an adequate inventory of species and knowledge of their range, distribution, habitat and biology.
To assess the ability of populations to survive habitat change, biological factors such as size distribution, reproductive age and rate, habitat specificity and dispersal ability are also important. The species most vulnerable to population decline through broad habitat change are those with a limited range and distribution consisting of localized inshore populations with a low reproductive potential. In respect to exotic diseases, it is often very difficult to identify those species most at risk.
The conservation significance of fish species, or definitions of fish biodiversity and marine bioregions, cannot be accomplished without knowing species diversity and distribution. For example, a recent publication by the Natural Heritage Trust, "Conservation overview and action plan for Australian threatened and potentially threatened marine and estuarine fishes" (Pogonoski et al 2002), was clearly compromised by the lack of information on fish diversity and distribution.
Further, advanced methods used to establish marine bioregions are currently based on statistical analysis of species assemblages including those of fish. Considering the resources put into these reports, and their use to direct resources in conservation programs, the lack of funding for an accurate assessment of fish diversity and distribution is remarkable. Many ecologists working on biodiversity programs are producing inaccurate reports based on models using unsubstantial data. Resources would be better spent through the collection of substantial data from the field.
The approach I made to elucidate the conservation knowledge of inshore demersal fish was to study, in detail, one fish group. This study included the group’s range and distribution, habitat and biology. The range of a species is a bio-geographical concept; for instance "the Deep-bodied pipefish is found from Port Phillip Bay, Victoria to Davenport Creek, South Australia". The distribution of a species is the patchiness, and nature of the patchiness, within its range "is restricted to the shallow low energy parts of bays or estuaries at Pelican Lagoon, Port Phillip Bay, St Vincent Gulf, etc.." or "generally distributed throughout its range". The habitat of species includes the geological, vegetation, faunal, and other components within its range. These may range from essential, to preferred or incidental. For a pipefish an essential component could be Zostera seagrass, a preferred certain depth where populations thrive and an incidental bryozoan which always grows on the seagrass, or mud or sand substrate.
Of the inshore groups to choose from, the larger reef fish are being systematically studied by divers in the program ‘Reefwatch’. Many large reef fish also have long life spans which can result in "living dead" populations where large old individuals are present but there is inadequate recruitment to maintain the population. Pelagic (migratory mid-water fish) are monitored by the department of Primary Industry and Resources (PIRSA) to determine the impact of commercial and recreational fishing. These species also generally produce many thousands of highly planktonic larvae which limits measuring the effect of local populations to recruitment. Often they spawn over a limited season which can result in population fluctuations due to poor conditions at the time. Their abundance, particularly at a local scale through migration or schooling behavior can be extreme, further limiting their value as small scale environmental indicators.
Therefore, candidate groups for this study were non-migratory demersal (bottom dwelling) fish, with small numbers of eggs, whose larvae had limited dispersal, and which had a high percentage of species living inshore in bays and estuaries. Previous studies suggested that the Syngnathids (seahorses, seadragons, pipehorses and pipefish) with possibly 50 species, most weedfish (30 species); and many gobies (40 species), snake-blennies and shore eels (20 species), and clingfish (20 species) could be suitable. I chose, of the Syngnathids, pipefish and pipehorses because of their popularity, a reasonable number of species and museum records, and the availability of literature.
The accuracy of taxonomics (the identification of and evolutionary relationships between species), and sampling type and effort, could bias an accurate conservation assessment of a species. Examples of sampling type are equipment used and the time of sampling; time of day, tides, seasons. Sampling effort includes the number of locations, the time spent sampling, and the amount and type of information recorded. Consequently, the pipefish were categorized by sampling types; inshore seagrass/rubble (0-2m; handnets or beach seines), shallow seagrass (2-20m; beam trawls), reef (1-20m; diving), and deepwater species (20-100m; commercial trawls).
Before the study could begin, an accurate key for pipefish identification that was easy to use was needed. Available keys were difficult to use so I produced an easy to use and accurate flow diagram-based key which reduced the number of pages for pipefish from 30 in the standard identification book to 10. I then checked whether the new key system worked with another group. Similarly the pages for weedfish (Heteroclinus) were reduced from 22 to 5. This type of key was based on three concepts and it could also easily have species removed to enable simplified regional keys to be made.
To familiarize myself with the species of pipefish and their confirmed distribution I consulted texts and ran through the collection at the South Australian Museum. To gain some practical field experience I handnetted a few locations to see how easy pipefish were sampled. I also established contacts with experts in fish from Victoria and Western Australia, and enquired if there were any studies or specimens of pipefish not included in current books or the museum records. To my surprise, in the literature there were few records of the size of maturity, fecundity (reproductive rate), or seasonality of reproduction in pipefish. In respect to conservation, these variables can tell you much about the reproductive potential of populations. The habitats in which reproducing and juvenile or sub-adults are found can also tell you the importance of different habitats to life stages. Because of the frequent questions I get asked about the reproduction of pipefish and the paucity of reports in the popular literature, I have included a short review of the current literature.
Unfortunately, the South Australian Museum is short-staffed when it comes to ichthyology. Studies of non-commercial marine fish species generally have to rely on a poor database. However, this is not the case for all groups and species. The ability of community organizations to contribute is shown by Native Fish Australia SA in their recent studies of the freshwater fish of South Australia. They have recently surveyed the South-east, Kangaroo Island and, to some extent, the River Murray and northern areas. A search of museum and other records were also made. Through this considerable effort, our knowledge of the diversity and distribution of South Australian fish has been greatly increased. Many species new to the state, or to regions, have been identified, and new species recognized. This has shown that conservation measures were inadequate for many species.
In spite of poor financing, the South Australian Museum has one of the top three programs for studying the molecular biology of fish in the world. This program enables the clear separation of species and of sub-populations. This knowledge combined with information about the range of species enables sound conservation practice. The molecular biology program also needs specimens of marine fish from throughout South Australia, from each species populations to distinguish phylogeny, species, sibling species (newly evolving species), unique populations and other information critical to conservation.
The unique sub-population of the famous giant cuttlefish in upper Spencer Gulf from others was shown by this method. Molecular studies with frogs in eastern Australia showed that what had appeared as one species was, in fact, several, enabling improved conservation management. These studies also infer that many species of previously unidentified frog species are already extinct. This is why the molecular analysis of South Australian fish is essential to their conservation.
On the shelves in the museum were several hundred bottles of pipefish. Many of these bottles contained one or two pipefish, but some contained more. The specimens were from a scattering of locations across South Australia. However, some collectors had left a legacy of locations where systematic collecting over time of a range of species had occurred. From each sample in the museum I recorded the location and date, checked the species, measured the size and sexual maturity and, if brooding, the number of eggs on males. For details of systematics, Dawson 1984 was consulted.

The invaluable collection of pipefish in the South Australian Museum. The collection includes samples of many other inshore demersal fish, many of which are unidentified, misidentified, or uncatalogued.

Robert observes pipefish under the microscope. Because of their small size microscope work is needed for some pipefish taxonomy. However, once species are confirmed simple keys can be used for their identification.
The SA museum database records the date, locality and species and sometimes notes on habitat. Many discoveries were made during the examination of the museum specimens. Some were mislabeled, some included more than one species and some were not included in the catalogue. This information from the SA Museum will soon be updated. Currently many databases from museums and other institutions are being incorporated into a national database. This database will be a powerful tool and will be accessible through the web showing a map of the recorded locations of any species and the specific museum record of any sample to be accessed.
As expected, I found that the extent of the museum collection and literature on any species of pipefish were largely dependent on its habitat. This is because the number of records of a species is a direct response to the type and ease of sampling. Previous to 1970 there were few pipefish collected by trawls in shallow seagrass. However, the efforts with beam trawls by Ward (1980) in the incidental collecting pipefish, a study of the effect of pollution on the benthic invertebrates at Port Pirie, and McDonald (2002) in a study of the fish of seagrass beds of Spencer Gulf had greatly increased the knowledge of shallow seagrass species and communities. Larger deepwater pipefish were regularly caught in trawls but smaller species may not have been detected. One interesting species sampled by trawling is the Tiger pipefish, Filicampus tigris. Three records of this species were taken in Spencer Gulf in the vicinity of Port Pirie and Whyalla. No specimens were known in museum databases until recently, in spite of some resurveying. In fact, the Tiger pipefish was listed as extinct in South Australia by Kuiter (2000), supposedly due to the effects of pollution on a small population. However, recently trawled specimens show the species is still extant. Another interesting type is Gales pipefish, Campithys galei, from WA, and Tryons pipefish, Campichthys tryoni. These are small species (7 cm) with only one supposed specimen of each found in South Australia. As Tryons pipefish has only been recorded from mid-north Queensland, whether the South Australian species is true remains uncertain. Species in this group have recently been sampled and further study may reveal the true status of this group.

A Tiger pipefish Filicampus tigris recently lodged at the Museum showed this species is not extinct in South Australia as previously suggested.
![]()
The Pygmy Pipehorse Idiotropiscis australe and many other cryptic reef dwelling species are best discovered by divers. New species have recently been found even in suburban Sydney Harbour, NSW. By observing these species, which are usually localized, divers have made substantial contributions to our knowledge of their behavior and reproduction.
The ability of marine naturalists to contribute to conservation was shown with reef and inshore/rubble species. Reef species which represent about 30% of potential species were hardly represented in collections and should be readily sampled by recreational divers. Even at the busy Clovelly Beach in Sydney’s eastern suburbs, the well-known underwater photographer Akos Lumnitzer found a new species of pygmy pipe-horse in 2003. However, only one of the reef species, the Southern Pygmy pipehorse, Idiotropiscis australe, is recorded in South Australia from Cape Jervis and St Vincent Gulf. Pygmy pipehorses are small (20-50mm long) and they camouflage themselves by growing appendages exactly matching the filamentous algae on reefs where they live. Pygmy pipehorses are distinguished from the protected seahorses and seadragons by their heads being in line with their bodies. Pygmy pipehorses are best observed at night and collected by divers sweeping a fine hand net through such habitat. Habitat preference suggests that many of the other reef pipefish found in Victoria and Western Australia could be found in South Australia.
The Spotted pipefish, Stigmatopora argus is found from NSW to Western Australia. Specimens of the Spotted pipefish vary considerably across this range. Consequently, new species could be identified within this complex. Systematic collecting from across southern Australia and the use of molecular analysis are needed to determine the taxonomic status of this group.



Gulf pipefish
Stigmatopora sp. nov.Interestingly, one of the Stigmatopora spp. complex, the Gulf pipefish Stigmatopora sp. nov?, is not officially recognized. However, as it was clearly identified from underwater photographs by diving marine naturalist Rudie Kuiter, a specific search was made for it in the museum. I found eleven unrecorded specimens and, if its authenticity is confirmed, the Gulf pipefish will be a new species endemic to South Australia. If this is the case, the Gulf pipefish is perhaps the rarest pipefish in Australia and one of particular conservation significance; 1) it has been recorded from only a few inshore few localities, 2) these are a rare mixture of rubbly/sandy bottom with seagrass in sheltered locations, 3) these are close to population centres, 4) because of its habitat it should be easy to find, 5) and none were found in offshore trawls which captured large numbers of other Stigmatopora sp. Specimens attributed to the Gulf pipefish have been recorded at Cape Jervis, Edithburg, Port Vincent, Port Victoria and Seacliff from 2-5m in association with rubble and seagrass Amphibolus. To increase our knowledge of this and other species, it is important that any specimens of the rarer pipefish found should be taken to the South Australian Museum (8207 7404, museum.info@saugov.sa.gov.au or Ralph Foster at foster.ralph@saugov.sa.gov.au) preferably live or, if not, frozen. There are only two pipefish with high snouts, the Knifesnout pipefish and the Gulf pipefish Stigmatopora sp. nov. If possible specimens of both should be taken to the South Australian Museum. Besides its high and wide snout the Gulf pipefish has a distinctive pattern on its belly.
![]()
This Knifesnout pipefish Hypselognathus rostratus was recently hand netted in less than one meter of water in seagrass at the Edithburgh Marina. This was the ninth specimen recorded. The lodging of this fresh specimen with the museum will its taxonomic status to be determined.
Most known inshore seagrass/rubble pipefish species were identified by marine naturalists in the 1800s or early 1900s using shore based methods, most probably hand nets. The early identification of these species by few individuals shows that simple sampling methods such as hand nets or beach seines are suitable for sampling of these habitats. Although the other inshore demersal fish have not been rigorously investigated, the same probably applies. Overall, pipefish records, except for deepwater trawled species, were mostly from locations near Adelaide, leaving large gaps in our geographical knowledge of pipefish. This particularly applies from Port Lincoln west past Ceduna, where virtually no records exist.
Vercos pipefish, Vanacampus vercoi, is a species that has been of conservation concern. This species was previously common in Pelican Lagoon on Kangaroo Island, and was recorded at both Point Turton and Sultana Beach on Yorke Peninsula, as well as a few dredged sites in Spencer Gulf. Thus it has one of the most restricted distributions of any pipefish. It was previously considered as two species, one of which has only been identified from Pelican Lagoon. At this stage, the systematics and distribution of this type are uncertain. Investigation of recently trawled specimens and molecular analysis of specimens from Pelican Lagoon and other locations are needed to establish the conservation status of Vercos pipefish.

Vercos pipefish Vanacampus vercoi is ENDEMIC TO South Australia and is considered as one of most localized in Australia. However, taxonomic work is required to tell if it is one or more species, and its relationship to similar species.
The efficiency of hand nets was shown by the diversity and number of pipefish species found. My hand net was one of the smallest prawn nets sold at fishing tackle shops, only 30cm diameter and of 3mm mesh. By netting Zostera seagrass for about one hour at low tide on 11 occasions, I was able to net about 200 pipefish from six species. Of course, most of these fish were released, after measuring and the determination of their breeding status. A few specimens from each location were vouchered for the confirmation of identification and molecular biology studies with the South Australian Museum. The efficiency of this simple, low impact and enjoyable method was recently shown at the Edithburgh marina. Forty minutes of netting yielded the ninth specimen of the Knifesnout pipefish, Hypselognathus rostratus, found in Australia and the first preservation of its genetic material. Also found were two crested weedfish, two spotted pipefish and an unknown goby. Inshore netting has also recently recorded the novel Gulf pipefish at Seacliff. Therefore, anybody willing to spend a few hours with a hand net in the shallows can contribute a great amount to the knowledge of, and conservation of South Australian fish.
‘For condemning their males to be paternal perambulators, the Syngnathidae have been termed the first suffragettes, although even those worthy women never went so far as to suggest that human fathers should be subjected to lying-in, and their modern sisters would scornfully expect the immediate extinction of Homo sapiens if the male of the species had to carry the baby’ (Whitley and Allan, 1958).
The reproductive biology of the Syngnathids is particularly interesting as the males brood the eggs. Seahorses have well developed brood pouches, seadragons have brood patches, and pipefish have brood patches which are enclosed to varying degrees. The brooding of eggs by males means that acceptance of the female by the male is a limiting factor in conferring genes to the next generation. This infers that the females would be advantaged by competing for males by ornamentation, pairing, courting or aggression, and all these activities have been observed
Courting is normal before mating in the Syngnathids, with complex courting rituals a pre-requisite to mating. In South Australia, females of both the Wide-bodied Stigmatopora nigra and Spotted pipefish court the males by displaying their chests which are barred. The chest of the Wide-bodied pipefish may be bright red. The South Australian Deep-bodied pipefish, Kaupus costatus has the greatest difference of form between males and females of any pipefish. The females are flattened sideways to display bright red and blue bars. Pairing is common in seahorses with pairs observed over long periods. However, pairing does not always mean fidelity as a wide range of mating patterns are documented in the Syngnathids, including genetic monogamy (faithful pairs) in a seahorse, and polygynandry (more than one female mate) and polyandry (more than one male mate) in pipefish (Jones and Avise 2001).
Recent studies using molecular techniques to elucidate paternity have shown that polyandry is common in some pipefish species. Moreover, in these species the intensity of sexual selection on females rivals that of any other animals (Jones et al 2001). This, and more females than males, with some females never reproducing in some species, results in very different behavior between males and females; the males are faithful and the females are dedicated, if promiscuous, partners. Male pipefish reject courting females other than their partner, maintaining the pair bond over seasons. This fidelity, and studies showing that even in primitive pipefish where external fertilization occurs, eggs exposed on the brood patch were all fertilized by the tending male, show the evolution of enclosed brood pouches is not a response to cuckoldry by sneaker males (McCoy et al 2001). Some have suggested that species which live amongst shelter which could brush away the eggs would have brood pouches. However, brood pouches are found in seahorses which do not violently interact with substrates, and brood patches in seadragons which have similar behaviours. Why then do advanced pipefish have a brood pouch? In these species, the embryos are attached to a placenta-like tissue which seals the pouch folds. The most apparent reason would be to reduce predation. However, no evidence exists of this. One study showed that within this enclosed pouch concentrations of salt were lower than in seawater (Watanabe et al 1999), perhaps reducing the energy expenditure from the egg needed for osmoregulation resulting in fitter larvae from similar sized eggs. If this is the case it is a further transfer of reproductive effort from the female to the male.
Mate guarding by females has been suggested as the main mechanism for maintenance of monogamy in males of pipefish; males losing their partners re-mate within a few days. Females will mate with other males besides their mate during a breeding episode (McCoy et al 2001). However, for unknown reasons widowed females remain unmated for a considerable period (Matsumoto and Yanagisawa 2001). Females in their quest to reproduce with as many males as possible have larger home ranges and are more active in courtship displays (Matsumoto and Yanagisawa 2001). Some interesting benefits have been shown from competition for partners in the pipefish. Broods from preferred matings when either males or females were allowed to choose a partner are superior at escaping predators and grow faster (Sandvik et al, 2000).
The number of eggs in most species of pipefish is not documented. A number of males from the South Australian Museum carried eggs or had mature brood pouches. This has enabled the first tabling of the fecundity of many species. A surprising find was that most species of pipefish only had between 20-30 eggs. This is in contrast to many seahorses which lay hundreds of eggs. In the Wide-bodied pipefish, reproduction had been shown throughout the year. However, there were few species with enough specimens over the seasons to show seasonality from the South Australian records. In many other species, the presence of brood pouches showed few males and in some species no males were found. Brood pouches could be subjected to seasonal variation or these samples could include only female or juvenile pipefish. If this is the case, other un-sampled habitat may be needed for reproduction. Further knowledge will be gained on reproductive status of individuals by their dissection to show the sex and the maturity of gonads.
Although in many pipefish, hatching time and the period between batches is not known, hatching time generally varies from 10-30 days. Pipefish can have several broods in a season, with non-brooding intervals as short as a few days (Matsumoto and Yanagisawa 2001). Many species have been shown to reproduce throughout the year (Howard and Koehn 1985). However, even closely related species may vary in respect to seasonality. The most likely reason for seasonality is variation of the potential adult food and larval supply.
This study shows that more knowledge is needed before sound decisions can be made about the conservation of pipefish in South Australia. Some species are clearly widely distributed and common. Of the inshore seagrass/rubble and shallow seagrass species, these include the Spotted pipefish, Wide-bodied pipefish, Pug-nosed pipefish, Pugnosa curtirostris, Long-nosed pipefish, Vanacampus poecilolaemus, and the Brushtail pipefish, Leptoichthys fistularius. Our current knowledge of some species suggests conservation concern because they are of limited distribution or little known (Vercos pipefish), widely distributed but localized (Deep-bodied pipefish, Kaupus costatus), localized and rare (Gulf pipefish), or are little known (Tiger pipefish, Gales and Tryons pipefish, Campichthys spp.). However, our knowledge of many other seagrass types is limited to a few specimens and sightings; for example the Knifesnout pipefish. Few specimens and sightings applies generally to reef species where there are few records and many novel species probably exist. This precludes any assessment of their conservation status.
There are a number of characteristics that are desirable in a fish group providing the best environmental indicators of inshore habitats; 1) easy sampling, 2) a moderate number of species, 3) restriction to limited inshore habitat, 4) a short lifespan, 5) reproduced over a long season, 6) produced few large offspring, 7) had a low dispersal rate. Although different species of pipefish varied in these characteristics, generally in different members of the group these characteristics were well represented.
It is clear that the community can greatly contribute, as they have in the past, to our knowledge of pipefish and other inshore demersal fish. Knowledge gained through this process will directly contribute to the conservation of the marine environment. This information can be used to formulate policy in respect to conservation and marine bioregions. Also, observing fish and the excitement of discovering novel species is fun and will provide individuals with an opportunity for environmentally constructive marine recreation.
Community contribution can be through observation or photographic records by divers; or by the collecting of specimens preferably fresh or frozen, and promptly lodged with the South Australian Museum. Once a reliable identification system is established through an extensive knowledge of species, there is no need for further vouchering of specimens. Collected fish can be reliably identified then released. A thorough knowledge of the species and their identification will enable the production of accurate regional keys and identification boards for divers.
Studies of the pipefish have shown that they, and other inshore demersal fish, are an ideal group for engagement of the community in both the diversity and distribution of fish, and for the monitoring of inshore habitat change. The need to monitor future changes in the inshore environment requires the establishment of a series of reference locations to be sampled regularly over the years. These locations should preferably have a mixture of substrates and vegetation types growing in shallow sub-littoral locations. In these locations a number of pipefish species could provide environmental indicators. As general indicators, these species include the Spotted pipefish and Wide-bodied pipefish. Habitat specialists of value include the Deep-bodied pipefish, Port Phillip pipefish and Gulf pipefish. In addition, a number of other inshore demersal fish could also be successfully sampled with the simplest of means such as hand nets or small beach seines. The community could easily contribute to this sampling and long-erm records from suitable locations would provide a wonderful opportunity for a field and web based marine conservation project.
The information in this essay and much more will be included in a document detailing the Sygnathid species of South Australia, and the current knowledge of their range, distribution, ecology, and biology. This document will be advertised through all marine societies and made publically available. A complimentary web site produced to facilitate community involvement in the proposed South Australian Fish Biodiversity Project will also be produced.
However, in synchrony with increased fieldwork is the need for adequate provision of resources by the government for the systematics of species, the adequate identification and record keeping of the museum collection and provision of information of our fish to the public. As things stand - reminiscent of a third world country - the museum workers often lack the funds for simple supplies such as specimen bottles and stationary. As a volunteer I had to provide my own felt markers. Therefore, besides contributing to conservation through observations, photographs or the provision of specimens, marine conservationists must also enthusiastically lobby the South Australian Government to redress this lamentable situation.
Acknowledgements:
Many thanks to the Marine Life Society of South Australian and the Scuba Divers Federation for their endorsement; and the Dept of Environment and Heritage, South Australian Museum, and the Dept of Primary Industry and Resources for their encouragement of this project. Particular thanks to Steve Reynolds (MLSSA) for the editing of this article, and Ralph Forster (SA Museum) and Micheal Hammer (Native Fish Australia SA) for their assistance, and the South Australian Museum for access to their Sygnathid collection and the provision of facilities. Interstate assistance was provided by Rudie Kuiter (Managing Editor, Zoonetics erBOOKS, www.zoonetics.com), Barry Hutchins (Western Australian Museum), and Martin Gammon (Museum Victoria).
References:
Dawson CE. 1985.
Indo-Pacific pipefishes: Red Sea to the Americas. The Gulf Coast Research Laboratory, Ocean Springs, Mississippi, USA.Howard RK, JD Koehn. 1985. Population dynamics and feeding ecology of pipefish (Syngnathidae) associated with eelgrass beds of Western Port, Victoria.. Aust. J. Mar. Freshwat. Res. 36(3): 361-370.
Jones AG, JC Avise. 2001. Mating systems and sexual selection in male-pregnant pipefishes and seahorses: insights from microsatellite-based studies of maternity.
J Hered. 92(2):150-8.
Jones AG, D Walker, JC Avise. 2001. Genetic evidence for extreme polyandry and extraordinary sex-role reversal in a pipefish. 1: Proc R Soc Lond B Biol Sci. 268(1485): 2531-5.
Kuiter RH. 2000. Seahorses, pipefishes and their relatives: a comprehensive guide to the Syngnathiformes. TCM Publishing, Chorleywood, UK.
Matsumoto K, Y Yanagisawa. 2001. Monogamy and sex role reversal in the pipefish Corythoichthys haematopterus. Anim Behav. 61(1):163-170.
McCoy EE, AG Jones, JC Avise. 2001. The genetic mating system and tests for cuckoldry in a pipefish species in which males fertilize eggs and brood offspring externally. Mol Ecol. 10(7): 1793-800.
Pogonoski JJ, DA Pollard, JR Paxton. 2002. Conservation overview and action plan for Australian threatened and potentially threatened marine and freshwater fishes. National Heritage Trust.
Sandvik M, G Rosenqvist, A Berglund. 2000. Male and female mate choice affects offspring quality in a sex-role-reversed pipefish. Proc R Soc Lond B Biol Sci. 267(1458): 2151-5.
Watanabe S, T Kaneko, Y Watanabe. 1999. Immunocytochemical detection of mitochondria-rich cells in the brood pouch epithelium of the pipefish, Syngnathus schlegeli: structural comparison with mitochondria-rich cells in the gills and larval epidermis. Cell Tissue Res. 295(1): 141-9.
Whitley G, J Allen. 1958. The sea-horse and its relatives. The Griffin Press, Adelaide.
By
Christopher B. Danielsa*, Sandra Orgeiga, Lucy C. Sullivanab, Nicholas Lingcd, Michael B. Bennette, Samuel Schürchf, Adalberto Luis Valg and Colin J. Braunerh
Running title: Air bladder surfactant in fish
Abstract
Several times throughout their radiation fish have evolved either lungs or swim bladders as gas holding structures. Lungs and swim bladders have different embryological origins and can be used either for buoyancy or as an accessory respiratory organ. Therefore, the presence of air-filled bladders in different groups of fishes, is an example of convergent evolution. We propose that air breathing could not occur without the presence of pulmonary surfactant and suggest that this system may have originated in epithelial cells lining the pharynx. We examined the surfactant system in swim bladders of three teleost fish (the air breathing pirarucu, (Arapaima gigas) and tarpon (Megalops cyprinoides) and the non-air-breathing New Zealand snapper (Pagrus auratus)). We related the presence and structure of the surfactant system to those previously described in the lungs of more primitive air breathing Actinopterygiians and Sarcopterygiians. We determined the presence of surfactant using biochemical, biophysical and morphological analyses and determined homology using immunohistochemical analysis of the surfactant proteins (SP). Snapper and tarpon swim bladders are lined with squamous and cuboidal epithelial cells, respectively, containing membrane-bound lamellar bodies. Phosphatidylcholine dominates the phospholipid profile of lavage material from all fish analysed todate
. The presence of the characteristic surfactant lipids in pirarucu and tarpon, lamellar bodies in tarpon and snapper, SP-B in tarpon and pirarucu lavage and the surfactant proteins (SP-A, -B and –D) in swim bladder tissue of the tarpon, provide strong evidence that the surfactant system of teleosts is homologous with that of other fish and tetrapods. This study is the first demonstration of the presence of SP-D in the lungs of non-mammals and SP-B in Actinopterygiian fishes. The extremely high Chol/DSP and Chol/PL ratios of surfactant extracted from tarpon and pirarucu bladders and the poor surface activity of tarpon lavage surfactant are characteristics of the surfactant system in other fishes. Despite the paraphyletic phylogeny of the fish, their surfactant is uniform in composition and may represent the vertebrate protosurfactant.* To whom correspondence should be addressed
Ph: 618-8303 6129
FAX: 618-8303 4364
Email:
chris.daniels@adelaide.edu.aua
School of Earth and Environmental Sciences, University of Adelaide, Adelaide, SA 5005 Australia;b
present address: Department of Microbiology and Immunology, University of Melbourne VIC 3010 Australia;c
Department of Zoology, University of Auckland, New Zealand;d
present address: Department of Biological Sciences, The University of Waikato, Private Bag 3105, Hamilton, New Zealand;e
School of Biomedical Sciences, Department of Anatomy and Developmental Biology, University of Queensland, St Lucia, QLD 4072;f
Department of Physiology and Biophysics, University of Calgary, Calgary, Alberta T2N 4N1, Canadag
The Instituto Nacional de Pesquisas d’Amazonia (INPA), Manaus, Amazonas, Brazil, 69083h
Department of Zoology, University of British Columbia 6270 University Blvd., Vancouver, BC V6T 1Z4 Canada;
Diet and feeding behaviour of the Blue-throated Wrasse
(published in Mar. Freshw. Res. 2001 Vol. 52, pp. 311-322.)
By Scoresby A. Shepherd and Peter Clarkson
This wrasse species was studied over a period of 15 years from 1984-1999 at West I. Juveniles were found to feed mainly on amphipods and small gastropods living in algae, and with increasing size the diet gradually shifted to crabs, large gastropods and shrimp. Several hundred preference experiments were done on the bottom and these showed that the most preferred prey were crabs and small abalone and shrimps, then gastropods, then ophiuroids with seastars lowest on the preference hierarchy. Normally the wrasse foraged for periods up to 10-15 min., and then retired to its crevice where it rested for some 5-10 minutes before coming out to forage again for food. Hence at any one time a diver is likely to see only about 60% of the population swimming around. The 15-year study examined the effect of wrasses on juvenile abalone and found that the consumption of little abalone at the study site was 30-70 abalone per year, depending on density. In other words the wrasses actually controlled abalone abundance. Of couse the wrasses also controlled crabs, another major predator of small abalone. So by controlling crabs, the wrasses ensured that crabs did not reduce abalone abundance too much. When seals arrived at the island in 1991, they reduced the number of wrasses by 70%, and the survival of little abalone improved substantially, thus demonstrating what is known as a cascade effect ie the abundance of higher order carnivores has a trickle down effect on species lower in the food web. The study demonstrated overall one of the values of protected areas, because it showed the strong interaction of different species up the food web.
I also monitored the abundance of the same wrasse at Cape Jervis over the same period of time. Here, due to intense coastal fishing, wrasse abundance declined, and in recent surveys males were never seen, indicating that shore fishers can severely affect populations of these wrasses by fishing the males (which preferentially take the bait), and in the long run cause a decline in numbers.
The sexual system is haremic, i.e. there are usually 10-15 females to each male. When the male dies, the largest female switches to a male and over about 2 months adopts the bluish breast and shape of the male. So intense fishing, by removing the males and large females, can affect reproductive success and reduce the number of small ones entering the population. This in turn can affect the abundance of crabs, abalone and probably many other species in a community.
Marine protected areas, by conserving the ecosystem in small areas can help conserve biodiversity, but can also tell us what are the subtle, long-term effects of fishing in coastal waters.
Blue-throated Wrasse on the Dredge at Glenelg
Photograph:
David Muirhead
By Steve Reynolds
Much has continued to happen since our nation’s celebration of "Encounter 2002". My article "After The Encounter" featured in at least six newsletters commencing with the January 2003 (No.295) issue. This new article discusses a few of the happenings to occur since "Encounter 2002".
As reported in our October 2002 Newsletter (No.293), in February 2003 the SA Maritime Museum put on permanent display the touring exhibition that had been travelling around SA. This exhibition includes specimens provided by our Society (as reported in our February 2002 Newsletter, No.285). Kevin Jones, the museum’s Director, invited me to view the exhibition. I was able to visit the Maritime Museum on 18th March 2003. The specimens did not appear to be set in resin. There were three separate displays showing some of the specimens provided. One of the displays just featured the Red Sea-Toad (Spider Crab), Schizophrys rufescens. Another display featured just Gastropod shells. These were the three Pheasant Shells, Phasianella australis, and the Black Cowry, Cypraea (Zoila) freindii thersites. A third display featured the two sponge species. I don’t know at this stage what became of the two starfish species that we provided. Each individual display credited the Marine Life Society. I also saw a display recognizing sponsors for the exhibition and that credited the Society as being one of the sponsors.
Little progress has been made regarding the search for the Casuarina’s lost anchor to date. At the Great Southern Dive Expo at Wirrina on 1st February 2003 Bill Jeffrey, a maritime archaeologist with the Department of Environment and Heritage (Heritage SA), confirmed that this project is on going, but making slow progress.
Late in 2002 the State Government held a competition through the Sunday Mail newspaper to choose a name for a small rocky island off Cape Blanche. The rocky outcrop is a seal sanctuary south of Streaky Bay on the west coast. The Sunday Mail held a "Name The Island Competition" and there were two joint winners. They both chose the name "Nicolas Baudin Island", a fitting tribute to Baudin during the bicentennial year of his expedition’s charting of our waters. The proposed name went before the Geographical Names Advisory Committee which invited public comment on the matter.
I decided it was necessary to put in a submission regarding the name because the Advisory Committee somehow decided that ‘Nicolas’ should have an ‘h’ in it. I told them that "I agree with the suggestion of naming an island after Nicolas Baudin but Baudin’s name was actually Nicolai. We anglicize his name by calling him Nicolas. If we are to use the name of Nicolas we should keep the ‘h’ out of it, i.e. Nicolas, NOT Nicholas. So I suggest that the exact name to be assigned to the island should be "Nicolas Baudin Island" (as suggested by the winners of the competition).
I contacted the Geographical Names Advisory Committee late in May 2003 whilst writing this article to enquire about the result of the public consultation but I was advised that no decision had yet been made or gazetted.
Remember Karom Thomasson, the French artist and sculptor, who created a giant crop sculpture near to The Bluff at Victor Harbor on March 22nd 2002? It was a 6ha artistic figure called "Cosmosapiens" (Child of the third millenium) that he created. Well, Adelaide film maker Matt Shannon made a movie of the same name (Cosmosapiens) about the giant sculptural figure. His film was the finale screening at the French Film Festival 2003 on 8th April 2003 (the 201st anniversary of the encounter between Flinders and Baudin).
Late in March 2003 former French Prime Minister, Michel Rocard returned to SA to celebrate the bicentenary of Nicolas Baudin’s circumnavigation of Kangaroo Island in 1803. On Thursday 27th March he visited the French-owned winery Orlando Wines at Rowland Flat in SA’s Barossa Valley accompanied by Anne Levy, the French consul in SA. They then had lunch at Jacob’s Creek Information Centre. The following day Mr Rocard unveiled a monument at Victor Harbor to commemorate the encounter between Flinders and Baudin in 1802.
Unfortunately, The Advertiser reported that the encounter occurred in 1803 and were unmoved when I phoned to inform them of their error. "It’s only one year out" they exclaimed. I should have insisted on a correction.
I don’t want to criticize The Advertiser too much because it is a great source of information for me. I am disappointed though at some of the errors that creep into reports. Take for example the "Our State" lift-out on 20th May. A time-scale of events over the past 400 years said that Flinders and Baudin met on April 9th in 1802, which they did, but the encounter first occurred on the 8th.
In order to gain more information about the memorial at Victor Harbor, I rang the National Trust’s Encounter Coast Discovery Centre in Flinders Parade (there’s that name Flinders again), near the causeway there. The Centre is open from 1 to 4pm every day except Christmas Day and Good Friday. It has both permanent and temporary displays (both audio and visual) concerning the encounter and it is open for extended hours during summer and school holidays.
They put me in touch with the local National Trust’s Chair, Mr. Wal Grant who explained that the memorial involved three poles in the causeway car park at Victor. He then sent me a copy of the local paper’s (The Times) 27th March 2003 report on the unveiling which gave the following details: -
"WHAT THE THREE POLES REPRESENT –
The forms are based on ships’ rigging, the introduced species of the Norfolk Pine, and the indigenous Knobby Club Rush.
1
Anemometer – instrument for measuring the force of the wind.2
Moiré – having a clouded appearance?3
Aeolian – of Aeolus, the god of winds.My thanks to Wal Grant for his assistance. He also sent me other copies of paper cuttings from 2002 and earlier concerning the encounter.
On Saturday 29th March Mr Rocard attended a community lunch on Kangaroo Island. Premier Mike Rann hosted a private dinner for Mr Rocard during his visit to SA.
In my "After The Encounter" article (Part 2, February Newsletter, No.296) I said that the 33m-long* brigantine Windeward Bound tall ship left Sydney Harbour on 7th March 2002 on a 16 to17-month commemorative voyage around Australia. The ship was sailing anti-clockwise around the country and was due to arrive in Port Adelaide on 19th April 2003. The ship actually arrived in Port Adelaide about one week early. I could see that a ‘different’ tall ship was in the Port as I drove over the Birkenhead Bridge. A news report on television confirmed that the Windeward Bound was in the river. I went down to the Port’s wharves on 13th April for a look at the ship. I was able to take a photograph and pick up a couple of pamphlets about the ship. She is a Sail Training Vessel, hence her full title is STV Windeward Bound.
*Her hull length is 24m. Her overall length of 33m includes her bowsprit, the spar running forward from her bow.
Sometime during the ship’s first week in Port Adelaide, a new book was launched onboard her. The book was titled "Sailing with Flinders – The Journal of Seaman Samuel Smith". The book’s details came from the journal of Samuel Smith who had been one of the 88 people onboard Flinders’ ship the Investigator. The book was edited by Dr Peter Monteath, a history lecturer at (appropriately) Flinders University.
The Windeward Bound left Port Adelaide on 8th May to sail to Victor Harbor. She then moored in Encounter Bay on Mother’s Day (11th May) and was open to the public for tours in the morning. The Seafarer II ferried people out to the ship. In the afternoon she spent two hours sailing around the bay.
Official Naming Of Nicolas Baudin Island
Early in June I received notification from the Supervisor of the Geographical Names Unit that the naming of Nicolas Baudin Island had become official as of 29th May. The Unit sent me a copy of the relevant page from The South Australian Government Gazette (2066, 29 May 2003). Jay Weatherill, as the Minister for Administrative Services, was the Minister responsible for announcing the assigning of the name. The name is assigned under the Geographical Names Act 1991. I was pleased to see that the name chosen used the spelling of Nicolas without an ‘h’. The island is just west of Cape Blanche, near Sceale Bay.
Dragon Search: Public Report (Abridged)
Summary of South Australian Sighting Data to January 2003
Prepared by
J.L. Baker,for the Dragon Search Community-Based Monitoring Project
Part 1 of this report discusses the sightings recorded by Dragon Search divers and beachcombers between January 1990 and January 2003. Additional historical records and undated records from Dragon Search reporters and the South Australian Museum are presented in Part 2 of the report. In most cases, specific locations at which live seadragons have been sighted are not discussed in this report, to preserve a confidentiality agreement made by Dragon Search with the divers who have provided information for the program. Site-specific details have been listed only for those localities that are well known and publicly promoted for viewing seadragons.
PART 1: Sightings January 1990 - January 2003
Both Species
: To 29th January 2003, 760 sightings have been recorded in South Australian waters for the Dragon Search Program, including 70 records in which leafies and weedies were recorded together, and 4 sightings when fish other than seadragons were seen. The number of records also includes, in some cases, repeat sightings over time of the same animals. To date, around 2198 seadragons have been reported in the main database (January 1990 – 29th January 2003), including a number of cases in which the same animals were recorded. A number of significant historical sightings have also been provided to Dragon Search, and are discussed in Part 2 of this report. The number of sightings per year recorded between 1990 and 2002 are listed below. To January 29th 2003, there have been 5 sightings reported so far in the 2003 year.|
Year |
No. Sightings |
|
1990 |
8 |
|
1991 |
24 |
|
1992 |
16 |
|
1993 |
8 |
|
1994 |
15 |
|
1995 |
46 |
|
1996 |
56 |
|
1997 |
92 |
|
1998 |
116 |
|
1999 |
130 |
|
2000 |
84 |
|
2001 |
77 |
|
2002 |
83 |
It is noted that the database contains one sighting of a "mass" of live leafies observed by a diver, and a number of reports of masses of dead leafies and weedies on beaches (predominately reported during the two "pilchard kill" events in SA waters during the mid-late 1990s). Such records can produce bias in calculations of the number of seadragons recorded according to variables such as month, location, depth, habitat type, sighting mode etc.
Both Species: The locations of South Australia’s 8 Marine Bioregions, as specified by the Commonwealth (IMCRA Technical Group 1998) are shown in Map 1.
The figures cited above are likely to reflect greater knowledge of the Dragon Search program amongst divers (and a relatively greater number of divers) in the more populated and accessible areas of South Australia, including popular dive spots around the metropolitan and southern metropolitan area; southern Fleurieu; Victor Harbor / Encounter Bay area; and Yorke Peninsula jetties; as well as the contribution of a number of records (including repeat sightings) from sites on Kangaroo Island where seadragons are regularly observed during dive tours.
There are also several beachcombers in the metropolitan and southern Fleurieu regions who consistently provide records, as well as a larger number of beachcombers in that region, compared with more remote locations. This accounts for the relatively high collective number of beachcombing records from several SVG Bioregion beaches in the metropolitan, southern metropolitan, and Encounter Bay areas.
Figure 1: South Australian Marine Bioregions (IMCRA 1998).
Weedies
: Of the 362 sightings in which weedies have been reported, 74% of the records have come from locations in the Gulf St Vincent (SVG) Bioregion (see above); around 12% have come from the Eyre Bioregion; around 5% from the Coorong Bioregion; 4% from the Otway Bioregion; almost 2% from the Spencer Gulf Bioregion, and less than 2% from the Murat Bioregion.Leafies: Around 89% of the 463 leafy sightings recorded to date have come from the SVG Bioregion; 7% of sightings have been recorded in the EYR Bioregion; around 1% each from the COR, MUR and SGF Bioregions, and less than 1% from the OTW Bioregion.
Weedies and Leafies Sighted Together: Around 90% of the 70 sightings of both species together have come from locations in the SVG Bioregion (mostly from Rapid Bay, accounting for 53 records, with 3 records from the Seacliff – Marino area; 2 records from Edithburgh, and one each from the Encounter Bay area; a bay off Innes National Park (Yorke Peninsula); a popular dive spot off the southern Fleurieu; a southern metropolitan beach area, and a site on the mid-northern coast of Kangaroo Island. For the Eyre Bioregion, 3 reports of both species sighted together came from the Anxious Bay region, and one report each from bays on southern Kangaroo Island. One record of both species came from the Murat Bioregion (Corvisart Bay / Back Beach area), and one from a reef site in the lower South East (Otway Bioregion).
Biounit Distribution of Sightings: Figure 1a below shows the distribution of seadragon sightings that have been recorded by Dragon Search between January 1990 and January 2002, within South Australian marine "Biounits". Biounits are coastal marine classification and planning units, devised by Ortiz (1992) for bioregional classification in NSW, and later applied to S.A. coastal marine areas by Edyvane (1999). Biounits in S.A. have been classified using coastal marine geomorphological and geological data, physiographic features, small spatial scale oceanographic features, and the distribution of some major benthic habitat types. Nominal biounit boundaries were set at 30m for the gulfs biounits, and 50m for oceanic biounits (see Edyvane 1999 for Biounit descriptions). The distribution in Figure 1a includes repeat sightings at the same locations.
Figure 1a
Biounit sightings in Figure 1a are arranged in approximate order from the westernmost biounit in which seadragons have been recorded (Wahgunyah, towards the WA border), to the easternmost biounit (Piccaninnie, in the South East of South Australia).
Between January 1990 and January 2003, seadragons have been reported from 25 of S.A.’s 35 biounits. Weedies have been reported from 24 biounits, and leafies from 19 biounits. In the southern Fleurieu Peninsula’s Yankalilla Biounit, almost 32% of all Dragon Search sightings have been recorded, particularly from the Rapid Bay area (from which 78% of the leafy sightings and 59% of the weedy sightings from that biounit have been recorded); another popular dive spot off the southern Fleurieu (11% of both leafy and weedy sightings from that biounit), and the Noarlunga area ( 9% of weedy sightings from Yankalilla Biounit).
The large number of sightings from popular dive spots, as well as repeat sightings from those specific locations (such as Rapid Bay) has influenced the summary statistics regarding numbers of seadragons sighted per biounit. Therefore, 35% of the total number of leafy seadragons recorded in the Dragon Search database, and 29% of the total number of weedy seadragons recorded, came from the Yankalilla Biounit.
Between January 1990 and January 2003, 14% of Dragon Search sightings have been recorded from sites in the Encounter Biounit, particularly from the Encounter Bay area (= 88% of sightings in that biounit, with the remainder from the surf beaches to the west and east, and 1 record from the bottom of the Fleurieu). Of the sightings in the Encounter Bay / Victor Harbor area, 73% of all sightings were dive records of live seadragons.
In the metropolitan area (Clinton Biounit), almost 15% of all seadragon sightings in the main Dragon Search database have been recorded. Around 32% of all sightings (= 31% of weedy sightings and 42% of leafy sightings) by divers in this biounit have come from a southern metropolitan reef area, and all but two records were from diving. Eighteen percent of Clinton Biounit records came from patch reefs and an artificial reef off the central metropolitan area, and 8% came from a northern metropolitan artificial reef (all diving records, and mostly of weedies). To January 2003, around 41% of seadragon sightings in this biounit have been beachwash specimens, mainly from beaches in the Henley, Brighton, and Tennyson area.
From north-eastern Kangaroo Island (Nepean Biounit), almost 13% of Dragon Search sightings have been recorded, and all but 1 of those records represent leafy sightings, mainly of single animals and pairs, reported from reefs and a jetty on north-eastern Kangaroo Island, including repeat sightings. The relatively large number of leafy sightings from that north-eastern area, reflects repeat observations of site-associated leafy seadragons from several locations. The single weedy sighting to date from the Nepean Biounit was a record from 1997, of an adult weedy caught whilst fishing, off the Dudley Peninsula area on Kangaroo Island.
To date, around 7% of all seadragon sightings in the Dragon Search database have been recorded from sites along eastern Yorke Peninsula (Orontes Biounit), the majority of which comprise sightings of leafies from jetty areas in south-western Gulf St Vincent. Very few weedies have been recorded by Dragon Search divers in the Orontes Biounit (i.e. 6 animals, from 5 sightings, between 1997 and 2002).
1. Sighting Details
Seasonal Summary of Sightings: Figure 2 below shows a monthly summary of seadragon sightings to January 21st 2003. Month was recorded for all sightings between January 1990 to January 2003. Excluding records in which seadragons were not seen, around 43% of all sightings were made during the summer months, 23% of sightings were recorded in autumn, almost 13% in winter, and 21% in spring. Similarly, for records by diving, snorkelling and other means (i.e. excluding beachwash records), 42% of sightings occurred in summer, 24% in autumn, 13% in winter and nearly 21% in spring.
Weedies: Around 40% of weedy sightings were made during the summer months, 26% of sightings were recorded in autumn, approximately 11% in winter, and a little over 22% in spring.
Leafies: Around 45% of leafy sightings were made during the summer months, 21% of sightings were recorded in autumn, almost 14% in winter and 20% in spring.
Figure 2: Monthly Summary of Seadragon Sightings, to January 2003
Neither relative frequency nor abundance of seadragons per sighting location can be meaningfully discussed on a seasonal basis due to the non-standardised nature of the recording, which is affected by a number of factors. These include (i) uneven distribution of recordings over space and time (i.e. areas were not systematically surveyed for seadragon presence, at all times of the year); (ii) individual preferences in the locations and seasons in which recorders chose to dive or go beach-combing (e.g. from late spring through to early autumn is a popular period for diving, because the water is warmer than at other times of the year, and summer is particularly popular, accounting for close to half of all the records by diving, snorkelling and other means); (iii) weather and/or sea conditions, and (iv) other opportunistic and/or uncontrollable aspects of the recordings. Apart from the smaller number of recreational dives that are taken in winter (which is a major factor biasing any seasonal summary of sightings, it is possible that after the breeding season, seadragons in some areas move offshore into deeper water (Kuiter 2000), which may also reduce the frequency of sightings during winter.
Despite these caveats, monthly distribution of seadragon sightings provides important supporting information when assessing seasonality of breeding, as discussed in the section below on Brooding Male Seadragons.
2. Habitat Details
To date, habitat type has been specified for around 76% of the 572 sightings by the applicable modes (day diving, night diving, snorkelling, other means), totalling 438 records, and 1069 seadragons, including repeat sightings in the same habitats, and also including several aggregate records, and sightings of large groups of seadragons. In the S.A. database, bottom type and habitat type are combined into a single field with overlapping categories, resulting in a number of different combinations of bottom type and habitat recorded. There appeared to be some lack of standardisation in the recording of bottom type and habitat details, and there were also a number of mixed habitat types and other habitats recorded. Percentages in the discussion below do not sum to 100 due to the overlap between habitat and bottom types, and the number of different combinations of habitat type recorded.
|
No. Weedy Seadragons per Group (including repeat sightings) |
Location |
Bioregion |
|
20 (18 were juveniles) |
Rapid Bay Jetty |
SVG |
|
16 (12 were juveniles) |
Rapid Bay Jetty |
SVG |
|
12 (2 records) |
Rapid Bay Jetty |
SVG |
|
12 (old record from November 1984, in historical database) |
An island in Encounter Bay |
SVG |
|
12 |
Noarlunga area |
SVG |
|
12 |
Carpenters Rocks - Blackfellows Caves area |
OTW |
|
11 |
Avoid Bay area |
EYR |
|
11 |
Artificial reef off a metropolitan beach |
SVG |
|
11 |
Rapid Bay Jetty |
SVG |
|
10 |
Rapid Bay Jetty |
SVG |
|
10 |
Site on mid-northern KI coast |
SVG |
|
9 |
Rapid Bay Jetty |
SVG |
|
8 |
Rapid Bay Jetty |
SVG |
|
8 |
Northern metro tyre reef |
SVG |
|
8 |
Southern metro tyre reef |
SVG |
|
8 |
An island in Encounter Bay |
SVG |
|
8 (old record from December 1979, in historical database) |
Seaford – Moana area |
SVG |
|
7 (3 records) |
Rapid Bay Jetty |
SVG |
|
6 (3 records) |
Rapid Bay Jetty |
SVG |
|
6 |
Carpenters Rocks - Blackfellows Caves area |
OTW |
|
6 |
A site 2 to 3 km east of Port MacDonnell |
OTW |
|
5 (6 records) |
Rapid Bay |
SVG |
|
4 (5 records) |
Rapid Bay |
SVG |
|
4 |
Carpenters Rocks - Blackfellows Caves area |
OTW |
|
4 (3 records) |
Victor Harbor area |
SVG |
|
4 (2 records) |
Avoid Bay area |
EYR |
|
3 (7 records) |
Rapid Bay |
SVG |
|
3 (2 records) |
Seacliff / Marino area |
SVG |
|
3 |
Site off northern metro beach |
SVG |
|
3 |
Site on mid-northern KI coast |
SVG |
|
3 (2 records) |
Avoid Bay area |
EYR |
3. Behaviour
To date, behaviour has been recorded for around 942 seadragons, including repeat sightings, and sightings of groups. No behaviour was recorded for around 31% of animals sighted by the applicable methods (day and night diving, snorkelling, or other sighting modes), including sightings of seadragon aggregations. The table below summarises the main behaviours observed for individuals and groups of seadragons, as a percentage of the sum of the number of seadragons for which behaviour was recorded.
|
Main Behaviour Observed |
% of Seadragons for which Behaviour was Recorded |
|
Hovering |
50 |
|
Hovering / Swimming |
14 |
|
Swimming |
13 |
|
Other |
11 |
|
Feeding |
6 |
|
Feeding / Swimming |
2 |
|
Feeding / Hovering |
1 |
|
Reproduction / "Egg Transfer" |
(1 record) |
4. Seadragon Groups and Singles
Groups of weedies have been recorded at a number of locations in several bioregions. The table below summarises the approximate locations of records of weedy groups, from 3 to 20 animals. It is possible that some of these records represent repeat sightings of the animal(s), or the same members of loosely structured seadragon groups, recorded either during the same day, or, in the case of some groups, within a few days of the previous dive. For example, recorded in the database are the following, amongst several other pairs or sets of dive records that appear similar:
5. Brooding Male Seadragons
Figure 3 following, summarises the number of sightings reported to date, of brooding male weedies and leafies, over the entire state.
Figure 3: Monthly Summary of Brooding Male Weedy and Leafy Seadragon Sightings
(N.B. Locations and Years Combined)
6. Juvenile Seadragons
To January 2003, juvenile weedies and juvenile leafies have apparently both been recorded throughout the year, except in September (with more records of juvenile weedies during summer and autumn than at other times of the year). Including beachcombing records, 47 records of juvenile weedies have been reported (19 of which came from Rapid Bay), and 85 records of juvenile leafies. Excluding records of old beachwashed specimens, which can add error to the distribution of months in which seadragons are of juvenile size, 41 juvenile weedy sightings have been
recorded, and 83 sightings of leafies. The larger number of sightings of juvenile leafies compared with weedies reflects (i) repeated diving during a two month period in 2001 at one site where juvenile leafies were observed (a popular dive spot in Encounter Bay); (ii) repeated diving during the summer of 1997-98 at a north-eastern Kangaroo Island dive site where juvenile leafies were observed; and (iii) regular diving at Rapid Bay when juvenile leafies were observed, during spring in 2001, and during summer to autumn, in 1996, 1999 and 2000, which collectively total 25 records of juvenile leafies from Rapid Bay.The tables following list the locations at which groups of juvenile seadragons (both with and without adults), have been observed, excluding beachwash records, which are discussed in a separate section.
|
Locations |
No. Weedy Juveniles |
No. Weedy Adults |
|
Rapid Bay Jetty |
18 |
2 |
|
Rapid Bay Jetty |
12 |
4 |
|
Rapid Bay Jetty |
4 |
2 |
|
Rapid Bay Jetty |
3 (2 records) |
1; 0 |
|
Rapid Bay |
2 (2 records) |
3; 3 |
|
Second Valley area |
2 |
0 |
|
Reef off a southern metropolitan beach |
2 |
0 |
|
Edithburgh Jetty |
2 |
0 |
|
Locations |
No. Leafy Juveniles |
No. Leafy Adults |
|
A dive site in Encounter Bay |
9 |
0 |
|
A jetty on south-eastern Yorke Peninsula |
5 |
8 |
|
Rapid Bay Jetty |
4 (2 records) |
6; 1 |
|
An artificial reef in north-eastern Gulf St Vincent |
4 |
0 |
|
Rapid Bay Jetty |
3 |
2 |
|
Rapid Bay Jetty |
2 (4 records) |
14; 2; 0; 0 |
|
A dive site in Encounter Bay |
2 |
0 |
|
A dive site on North-Eastern Kangaroo Island |
2 (2 records) |
0 |
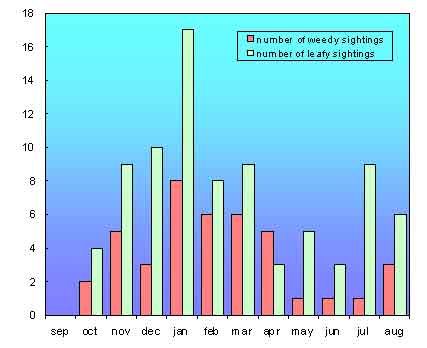
Figure 4: Monthly Summary of "Juvenile" Weedy and Leafy Seadragon Sightings
(N.B. Locations and Years Combined, and Excluding Records of Old Beachwashed Seadragons)
|
Date |
Location |
Beachwash Seadragons |
Comment on Sighting Form |
|
December 1999 |
Sceale Bay |
5 adult weedies |
"Specimens washed up with pilchards, unsure of exact numbers; found during neap tide after storm" |
|
December 1999 |
Corvisart Bay ("Back Beach") |
7 adult weedies |
"Specimens washed up with pilchards; two were gravid" |
|
December 1999 |
Black Point, Kangaroo Island |
19 adult weedies |
"Found washed up with pilchards and the odd puffer fish after stormy conditions the day before" |
|
February 1999 |
South Brighton |
1 adult leafy |
"Found washed up in a pilchard die-off wash". |
|
January 1999 |
D’Estrees Bay, Kangaroo Island |
4 adult leafies |
"Washed up with pilchards". |
|
January 1999 |
Goolwa |
2 adult weedies |
"Found amongst dead pilchards (old specimens also) and puffer fish, above high tide mark, in soft dry sand" |
|
January 1999 |
Corvisart Bay ("Back Beach") |
31 adult weedies |
"30 old, 1 fresh specimen(s) (approx. 23 male, 8 female); lots of dead pilchards with seadragons; would suggest that fish had been washed up on previous highest tide - 1/1/99 - many would have been collected by other beach combers" [i.e. prior to this sighting] |
|
December 1998 |
Between Tennyson and Grange jetty |
6 adult leafies |
"Sightings during approx. 2 week period during pilchard kill 1998. Also notable was pink blush on bodies of seadragons as on pilchards". |
|
December 1998 |
Between Grange and Henley Beach |
1 adult weedy |
"30cm long. A lot of dead pilchards on beach" |
|
December 1998 |
Parsons Beach |
12 adult weedies |
"Found amongst dead pilchards at tidal rim, many 'spiky fish' where seadragons found. Seadragons found at edge of tidal rim. Informed by friend that 10 seadragons were collected on same beach over a 2 day period". |
|
December 1998 |
Waitpinga Beach |
1 adult weedy |
"30cm long; at time of pilchard kill, found at high tide mark on sandy beach" |
|
December 1998 |
Waitpinga and Parsons Beach |
6 adult weedies |
"Of 6 specimens, 3 were in good condition, but all old specimens in dry sand - high up on the beach - edge tide line" (Note: dead pilchards with beachwash seadragons were sited in this region previous week – see other records for area) |
|
November 1998 |
Head of Great Australian Bight |
7 adult weedies |
"Coincided with pilchard kill". Reporter stated that "other sightings were recorded from the Point Bell location". |
|
November 1998 |
Flour Cask Bay, Kangaroo Island |
7 adult weedies |
"Masses of pilchards, brine shrimp, 6 crabs, 2 scorpion fish, 2 globe fish, 1 baby pilot whale; were all washed up with pilchard beach-washings". |
|
November 1998 |
Venus Bay |
2 adult weedies |
"Masses of pilchards; 3 seahorses (all fresh; one black and white striped) washed up 1 week after mass pilchard death". |
|
October 1998 |
Henley Beach |
1 adult weedy |
"Lots of dead pilchards all the way along the beach". |
|
July 1996 |
Frenchman’s Beach (Coffin Bay area) |
75 adult leafies |
"Time of pilchard deaths". |
|
July 1996 |
Fishery Bay (southern Eyre Peninsula) |
2 adult leafies |
"50-100 dead leafies at Frenchman's Beach at same time. Time of pilchard deaths". |
7. "Beachwash" Seadragons
To date, 188 sightings of beached seadragons have been recorded, comprising a total of 828 specimens. There is also 1 beachwash record of a mass of sea urchins and sea stars washed up at Emu Bay (Kangaroo Island) after a storm, and an additional 5 records of beachwashed specimens for which a sighting mode other than beachcombing was recorded. Almost 47% of all beachcombing sightings to date were recorded during the summer months; 21% during spring; 21% during autumn, and the remainder during the winter months. Around 55% of the sightings to date have been of fresh seadragons and 49% refer to old specimens, and the total of these two exceeds 100% because both fresh and old seadragons were recorded in 10 sightings (5% of beachcombing records). Fresh dead seadragons refer to recent beachwash specimens which are not shrunken or dried, are still colourful, and usually still have the appendages intact. Old specimens refer to dried, shrunken and/or decomposing seadragons. Almost 73% of beachwash records to date are of single specimens, although there are reports of pairs (11% of beachwash records). One sighting of a live beached seadragon has been recorded to date, of an adult weedy recorded at Tennyson Beach, in September 1995
. The database also includes a small number of aggregated beachwash sightings presented as single records; for example 9 adult weedies and 1 adult leafy recorded at the Sellicks - Aldinga Beach area in 1992, over the month of January.There are records of small groups of seadragons or single specimens being washed up on beaches, after high tides, storms and/or large swells, and around 15% of beachcombing records specified such details in the section on other information. Examples include the following, amongst other records specifying storms, swells and/or high tides:
In recent years there have been two periods in which mass deaths of pilchards occurred in southern Australian waters. The first, in late 1995, commenced in the Anxious Bay area. The following winter, a mass stranding of around 75 dead leafy seadragons was observed south of Anxious Bay, at Frenchman’s Beach on the Coffin Bay peninsula. Many beach records of dead seadragons (including "mass strandings") in the database also coincide with the pilchard death event of late spring 1998 to late summer 1999, believed to be viral-related (Ward et al. 2001). Almost half (i.e. 48%) of all beachwash records were reported in 1998 and 1999, and many of the records from these two years specified that the seadragons were washed up with pilchards, or that the sighting occurred during the time of the second recorded mass pilchard die-off in S.A. waters (during the summer of 1998-99). Beachwash sightings for which dead pilchards were also recorded with the seadragons, include the following, in reverse chronological order.
There were a number of additional records of seadragon deaths (including mass numbers) that occurred during the timing of the second recorded pilchard kill event along the South Australian coastline, but those records did not specify pilchards in the beachwash. One example is 40 dead weedies found on a beach in the Encounter Bay area, in November 1998.
Beachwashed seadragons have been recorded from more than 60 locations along the South Australian coast. The tables below show some of the main locations in each Bioregion where beachwashed specimens have been recorded.
8. Other Data (Depth of Sightings; Water Temperature)
To date, 188 reports of weedy sightings have provided depth recordings. Of those reports, the recorded depth range of weedy sightings by divers and snorkellers collectively, has been 1m to 22m. Around 75% of the sightings occurred in waters between 3m and 12m; around 12% of sightings occurred between 18m and 20m diving range, and few sightings were recorded at other depths within the range 1m – 22m. For weedy sightings, there have been only 2 records by snorkellers for which depth data were provided, these being sightings at 4m and 9m.
Similar caveats apply to the interpretation of temperature recorded during seadragon sightings, particularly due to (i) the prevalence of diving from late spring to early autumn, (i.e. pleasant diving conditions), especially during the summer holiday months, and (ii) the under-representation of winter sightings. Of the records for which date was recorded, around 42% of sightings by diving, snorkelling and other means (excluding beachcombing) were recorded in summer; 24% in autumn, and 21% in spring, compared with only 13% in the winter months. To date, 104 records of weedy sightings have reported water temperature, which has reportedly ranged, to date, from 11oC to 24oC. Within the range, around 73% of weedy sightings for which temperature was recorded, were made in waters between 16oC and 22oC, with few records at cooler temperatures, due to preference for late spring, summer (particularly) and early autumn diving conditions. To date, the highest reported water temperature in which weedy seadragons were sighted was 24oC, purportedly at 17m depth at a reef off the Glenelg area, in March 1999. The lowest temperature in which weedy seadragons have been sighted to date was 11oC, supposedly recorded at an artificial reef off the northern metropolitan coast (at 19m) in August 2000.
9. Sites of Particular Note
Apart from the geographical limits of seadragon sightings by the Dragon Search program discussed in Section 2 (Bioregional Distribution of Sightings), other sightings of particular importance include the following:
Jetties: There is a disproportionately high number of reports from diving at jetties (246 records, representing 19 jetties), due to repeat sightings at popular dive spots where seadragons are known to occur, such as Rapid Bay Jetty (from which around 61% of all jetty reports have come); a jetty on north-eastern Kangaroo Island (14%), and Edithburgh Jetty on Yorke Peninsula (7%). A number of seadragons and seadragon groups are known to be "site-associated" with such jetties and are therefore regularly recorded by divers. Despite this bias, it is clear that jetty structures in various parts of South Australia, particularly the lower Fleurieu and lower Yorke Peninsula (Gulf St Vincent side) provide important additional 3-dimensional habitat for seadragons of both species. There have been a number of sightings from two of the jetties on south-eastern Yorke Peninsula (8% of jetty sighting records), and jetties in the Encounter Bay area (nearly 5% of all jetty sightings). Some of the less commonly dived jetties where seadragons have been recorded include jetties in:
Artificial Reefs: It is notable that artificial reefs (both constructed reefs and shipwrecks) appear to be part of the habitat utilised by seadragons, and weedies in particular have been noted on tyre reef and wrecks in various parts of the S.A. marine environment. For example, a total of 31 records (most of which refer to weedies) have been reported to date, from artificial tyre reefs in the southern (11 records), central (10 records) and northern (9 records) metropolitan coastal area.
A number of records have also come from wrecks off the central and southern metro coasts (3 records).
Those structures in the northern SVG area may be particularly important as additional seadragon habitat, given the pollution-induced decline in natural habitat that has occurred in the area during the past few decades.
PART 2: Historical Data
Dragon Search sightings pre-January 1990
The majority of records in the South Australian database to date, have been collected between 1995 and 2002, and all records from January 1990 onwards are discussed in Part 1 of this report. All records older that 1990 have been removed to a separate database, and to date, there have been 94 such records submitted by Dragon Search reporters, as well as "aggregate" reports for sightings over a number of years Summaries of the historical records are provided below:
Gulf St Vincent (SVG) Bioregion
Around 75% of the 97 historical Dragon Search records have come from the SVG bioregion, discussed below by date:
|
1989 Rapid Bay
A jetty in Encounter Bay
A site on the north-western coast of Kangaroo Island
Second Valley area
Reef off a southern metropolitan beach
|
1988 Rapid Bay
A jetty in Encounter Bay
A dive site in Encounter Bay
Wright Island, Encounter Bay
A reef in Encounter Bay
A site east of Sturt Bay, southern Yorke Peninsula
|
|
1987 Rapid Bay
Jetty in Encounter Bay
A dive site in Encounter Bay
A site on the mid-northern coast of Kangaroo Island
Edithburgh, Yorke Peninsula
A bay on southern side of Innes National Park, Yorke Peninsula
|
1986 A dive site in Encounter Bay
Second Valley area
|
|
1985 Encounter Bay
Encounter Bay
|
1984 Rapid Bay
Island in Encounter Bay
Seaford – Moana area
|
|
1983 Seacliff area
|
1982 Cape Jervis area
Seacliff area
|
|
1981 Encounter Bay
Seacliff area
|
1980 Rapid Bay
Island in Encounter Bay
Dive site in Encounter Bay
Seaford – Moana area
Hallett Cove beach
Jetty on north-eastern Kangaroo Island
|
|
1979 Encounter Bay
Seaford – Moana area
|
1978 Encounter Bay
Port Noarlunga area
|
|
1977 |
1976 Semaphore Beach
|
Historical Records from the SA Museum
Apart from the above historical records included in the South Australian Dragon Search database, the South Australian Museum provided 72 additional historical records to Dragon Search, which describe an assortment of leafy and weedy seadragon sightings from around South Australia.
The following summarises the SA Museum records (almost all of which were beachwash specimens), according to location, from west to east. Of particular note is the record of a weedy and 3 leafies recorded in 1985, caught live in a trawl at 12m – 20m, from Douglas Bank in upper Spencer Gulf, as this appears to be the only record known of seadragons occurring so far north in Spencer Gulf.
|
Location / Marker |
Bioregion |
Sighting Details for Records of Beachwash Specimens |
|
"West Coast of SA" |
|
|
|
Coffin Bay National Park |
EYR |
|
|
Port Lincoln |
EYR |
|
|
Tumby Bay |
EYR / SGF |
|
|
Neptune Island |
EYR |
|
|
Cape du Couedic, KI |
EYR |
|
|
D’Estrees Bay, KI |
EYR |
|
|
Douglas Bank, upper Spencer Gulf |
USG |
|
|
Wallaroo |
SGF |
|
|
Port Minlacowie |
SGF |
|
|
"Gulf St Vincent" (locations unspecified) |
|
|
|
"Wattle Point", Yorke Peninsula |
SVG |
|
|
Stansbury |
SVG |
|
|
Largs Bay |
SVG |
|
|
Semaphore |
|
|
|
Port Adelaide area |
SVG |
|
|
Grange area |
SVG |
|
|
Henley Beach |
SVG |
|
|
Glenelg |
SVG |
|
|
Brighton |
SVG |
|
|
Marino Rocks / Marino |
SVG |
|
|
Aldinga |
SVG |
|
|
Sellicks Beach |
SVG |
|
|
Normanville |
SVG |
|
|
Encounter Bay |
SVG / COR |
|
|
Port Elliot |
SVG / COR |
|
|
Coorong |
COR |
|
|
Robe |
OTW |
|
|
Location / Marker |
Bioregion |
Sighting Details for Records of Beachwash Specimens |
|
(unspecified locations within South Australia) |
Freshly dead leafies: Freshly dead weedies:
Old weedies:
|
Acknowledgements
The Dragon Search program thanks the various organisations, government programs and companies listed on page 1, which have supported and/or promoted the Dragon Search program in South Australia over a number of years. Particular thanks go to Anthony Flaherty (MCCN), Vicki-Jo Russell (TSN) and the
Marine Life Society of South Australia for ongoing support of this program since its inception, and to the divers and beachcombers who have contributed to the Dragon Search database over the past decade. We are especially grateful to those divers and beachcombers who have regularly monitored seadragons in particular locations, and have submitted their records to Dragon Search.Jeremy Gramp from Dragon Search, Anthony Flaherty from the Marine and Coastal Community Network, and Vicki-Jo Russell from the Threatened Species Network, have worked for a number of years to promote and develop the program throughout South Australia, and to assist the co-ordination of Dragon Search programs in other southern States. Jeremy also entered most of the data in the SA Dragon Search database, maintained the database, checked sighting details and contacted Dragon Search divers and beachcombers for additional information where required. The quality of this report has thus been greatly assisted by Jeremy’s efforts.
References
Australian Museum (2002).
Australian Museum Fish Site. http://www.amonline.net.au/fishes/Carrick, N. (1997). A Preliminary Assessment of the By-Catch from the Spencer Gulf Prawn Fishery. South Australian Fisheries Assessment Series 97/02. SARDI Aquatic Sciences, South Australia.
Cheshire, A. and Westphalen, G. (2000). Assessing the status of temperate reefs in Gulf St Vincent IV: Results of 1999 survey. A Report to the Environment Protection Agency of South Australia.
Cheshire, A., Hall, S., Havenhand, J. and Miller, D. (1998). Assessing the status of temperate reefs in Gulf St Vincent II: survey results. Report prepared for Environmental Protection Authority, SA. Botany Department, University of Adelaide, South Australia.
Connolly, R., Melville, A. and Keesing, J. (2002). Abundance, movement and individual identification of leafy seadragons, Phycodurus eques (Pisces: Syngnathidae). Marine and Freshwater Research 53: 777-780.
Edgar, G. (2000). Australian Marine Life. Revised edition. Reed New Holland, Australia.
Edyvane, K. (1999). Conserving Marine Biodiversity in South Australia – Part 2 – Identification of Areas of High Conservation Value in South Australia. SARDI Report Number 39. SARDI Aquatic Sciences, and Primary Industries South Australia.
Environmental Protection Agency (EPA) (1998). Changes in Seagrass Coverage, and Links to Water Quality off the Adelaide Metropolitan Coastline. Report by EPA, Department for Environment and Heritage, South Australia.
Environmental Protection Authority (2003). The health of subtidal reefs along the Adelaide metropolitan coastline 1996 – 1999. Report by S. Gaylard, Environmental Protection Authority (South Australia), based upon the results of the Reef Health project (see Cheshire et al 1998).
Froese, R. and Pauly, D. (eds.) (2002). FishBase. World Wide Web electronic publication. www.fishbase.org
Hart, D. (1996). Near-shore seagrass change between 1949 and 1995 – mapped using digital aerial ortho-photography – Northern Metropolitan Adelaide area: Largs Bay – Glenelg. Image Data Services, Resource Information Group, DENR, Netley SA.
Hart, D. (1997). Near-shore seagrass change between 1949 and 1996 – mapped using digital aerial ortho-photography – Metropolitan Adelaide area: Largs Bay – Aldinga, South Australia. Image Data Services, Resource Information Group, DENR, Netley SA.
Hutchins, B., and Swainston, R. (1986). Sea Fishes of Southern Australia. Swainston Publishing, Perth, Western Australia.
IMCRA Technical Group (1998). Interim Mariner and Coastal Regionalisation for Australia: an ecosystem-based classification for marine and coastal environments. Version 3.3. Environment Australia, Commonwealth Department of the Environment, Canberra.
Kuiter, R. (1993 and 2000a). Coastal Fishes of South-Eastern Australia. Gary Allen Pty Ltd., Sydney, Australia.
Kuiter, R. (1996a). Guide to Sea Fishes of Australia. New Holland Publishers Australia Pty Ltd. 430 pp.
Kuiter, R. (1996b). The Complete Divers’ Guide to Coastal Fishes of South-Eastern Australia. Natural Learning Titles, CD ROM.
Kuiter, R. (2000). Seahorses, Pipefishes and Their Relatives. A Comprehensive Guide to Syngnathiformes. TMC Publishing, Chorleywood, UK. 240pp.
Kuiter R. (2001). Revision of the Australian seahorses of the genus Hippocampus (Syngnathiformes: Syngnathidae) with descriptions of nine new species. Records of the Australian Museum Vol. 53.
Ortiz, E. (1992). The development of a representative system of marine and estuarine protected areas for New South Wales. Unpublished report by New South Wales Fisheries, for the Australian National Parks and Wildlife Service, Canberra. 37pp.
Shepherd S. (1970). Preliminary report upon degradation of seagrass beds at North Glenelg. Unpublished report. South Australian Department of Fisheries. 29pp.
Shepherd, S., McComb, A., Bulthuis, D., Neverauskas, V., Steffensen, D. and West, R. (1989). Decline of seagrasses. In: Larkum, A.., McComb, A., and Shepherd, S. (eds.). (1989). Biology of Seagrasses, Elsevier, Amsterdam.
Smith, N. (2000). The impact of the mussel Brachidontes erosus (Mytilidae) on subtidal South Australian macroalgal systems. Honours thesis, Department of Environmental Biology, University of Adelaide, South Australia.
Turner, D. and Cheshire, A. (2002). Effect of dispersed sediment plumes from beach sand replenishment dredging on recruitment of phaeophycean algae to rocky reefs in Gulf St Vincent, South Australia: final report incorporating surveys from 1998 – 2001. Department of Environmental Biology, University of Adelaide, S.A.
Ward, T., Hoedt, F., Mcleay, L., Dimmlich, W., Kinloch, M., Jackson, G., McGarvey, R., Rogers, P. and Jones, G.K. (2001). Effects of the 1995 and 1998 mass mortality events on the spawning biomass of sardine, Sardinops sagax, in South Australian waters. ICES Journal of Marine Science 58: 865-875.
Womersley, H.B.S. (1987). The Marine Benthic Flora of Southern Australia, Part II. Handbook of the Flora and Fauna of South Australia. Government Printer, Adelaide, S.A.
Patterns of movement and habitat use by leafy seadragons tracked ultrasonically.
By Rod M. Connolly, Andrew J. Melville and Keith M. Preston (2002).
Patterns of movement and habitat use by leafy seadragons, Phycodurus eques (Pisces: Syngnathidae), tracked ultrasonically.
Journal of Fish Biology, vol. 61, pp 684-695. School of Environmental and Applied Sciences, Gold Coast Campus, Griffith University
ABSTRACT
The leafy seadragon (Phycodurus eques Günther) is a relatively large member of the fish family Syngnathidae, and is an important conservation icon in southern Australia, where it is endemic.
Nine adult seadragons were tracked using ultrasonic telemetry for between 2-10 days around West Island.
Study Area
All fish except one moved within well-defined home ranges of up to 5 ha (using minimum convex polygon method3). Short bursts of movement (at average velocities of 2-17 m h-1) punctuated long periods (up to 68 h) without movement.
The exceptional fish moved almost in a straight line away from its tagging location near the end of the tracking period, at a maximum velocity of 146 m h-1.
There was no constant diel pattern2 in movements; some fish moved more at night, others during the day.
The time seadragons spent over particular habitats compared to the area of those habitats available at the study site was greater for Posidonia seagrass, about as expected for kelp-covered reefs and bare sand patches, and less than expected for Amphibolis seagrass and boulders covered with brown algae.
In searching for tagging effects, a comparison of movement immediately after tagging showed no difference with subsequent movements for most fish. The lack of tagging effect may be because the transmitter can be attached to the bony appendages away from the body of the fish. There was no sign of damage to fish upon removal of transmitters after tracking.
The measurements of movement and habitat use by leafy seadragons will assist in the design of marine protected areas where the species is a key consideration in management.
NOTES
Here is a detailed list of the major articles that have appeared in the MARIA and MLSSA Journals since they started in October 1979. Copies of the Journals are held in the MLSSA Library and may be either borrowed (if there are multiple copies, by members only) or a photocopy obtained (members and non-members) for a modest charge.
MARIA Journals
Volume 1 No. 1
October 1979
"The Fish of the Noarlunga Reef"
"Report on the Wreck Moorara"
Volume 1 No. 2
November 1979
"Transection at Victor Harbor"
"The Electric Organs in Fishes"
Volume 1 No. 3
January 1980
"The Shape of Seashells"
"Venomous Marine Invertebrates Lethally Dangerous to Man"
"The Size of Scallops"
"Murex Shell Growth"
Volume 1 No. 4
May 1980
"Symbiotic Algae in Clam Mantle Tissue"
"Stingose - A Treatment for Bites and Stings"
"First Aid for Jellyfish Stings" Geoff Mower
"Relief of Pain From the Box Jelly Carybdea rastoni Found in South Australian Waters"
"Notes on Keeping Some Temperate Marine Fish"
"Review of the Article - "Marine Reserves in South Australia - Proposals""
Volume 1 No. 5
September 1980
"Victor Harbor, The Bluff, Flora and Fauna Surveys 1980"
"The Colour of Fish"
"The Life Cycle of the Seagrass Amphibolus antarctica"
"The Higher Molluscs"
"The Blue Ringed Octopus"
"Asteroids (starfish) in South Australia
"Fish Profile - The Ornate Cowfish"
"The Redcliffe Issue"
Volume 2 No. 1
March 1981
"Mapping the Bottom Communities of Upper Spencer Gulf"
"Water Flow Patterns Through an Undergravel Filter Substrate Bed"
"Effects of Air Flow and Tube Dimensions on Water Flow Through Air-powered Uplift Pipes"
"Preliminary Experiments in Water Movement Through Air-powered Aquarium Uplifts
"A Mathematical Model for the Destruction of Seagrass by Freshwater and Effluent"
"Close-up Photography of Marine Life in the Aquarium, with a Still Camera"
"Fish Profile - The Old Wife"
"Key to Some Common Sea Stars"
"Marine Aquaria: A Source of Enquiry in Science Education"
"The Impact of Oil on the Marine Environment"
Volume 2 No. 2
December 1981
"The Artificial Reefs of South Australia"
"Seahorses"
"A Pictorial Guide to Some Common Caulerpa Species"
"Mathematical Model of Sandpatch Formation Due to Effluent Discharge"
"Biscuit Stars in the Aquarium"
"Maintaining an Ecological Balance in an Aquarium"
"Fish Profile - The South Australian Cobbler"
MLSSA Journals
No. 1
February 1985
"South Australian Museum Fish Survey"
"The Seacliff Wave Recorder"
"The Lateral Lines of Fish"
"Hermit Crabs"
"Fish Profile - The Smooth Toadfish"
No. 2
August 1991
"The Leafy Seadragon"
"Seadragon Sightings"
"Notes On Parental Care, Hatching and Raising of Seadragons"
No. 3
December 1992
"Our Society’s Concern Over the Effects of Oil Spills"
"The Occurrence of Chemical and Oil Spills in Upper Spencer Gulf in 1992"
"Upper Spencer Gulf Marine Life"
"Fish Species Sighted in the Upper Spencer Gulf"
"Information on Oil and Oil Spills"
No. 4
December 1993
"Glenelg-Port Adelaide-Bolivar Sewage sludge Pipeline Opened"
"The Sea Daisy"
"The Breeding of Sharks, Stingrays and Skates"
"The Aftermath of Oil Spills"
"Petrol Lead Levels and Sewage Nutrients"
"Propeller of the Port River"
No. 5
December 1994
"The Latest Events Concerning Sewage and Stormwater Discharge"
"More on Seadragon Sightings"
"Seadragon Monitoring"
"Science and Environmental Education Based on Beach Studies"
"Fish Sightings At Port Noarlunga Reef"
No. 6
December 1995
"The Origins of Names Associated With Victor Harbor"
"Marine Life Studies At Victor Harbor"
"The Plants and Animals of the Screwpile Jetty"
"Seadragon Sightings at Victor Harbor"
"Seadragon Reference List"
"Special Habitat Supplement - ACF"
"Ghost Pipefish"
"Northern Pacific Seastar"
No. 7
December 1996
"Seagrass Study"
"Ecology of Toxic Algae in the Port River"
"Fish Profile - The Moonlighter"
"Biological Filtration Experiment"
"Marine Reserves"
"Seahorses"
"MLSSA Photo Index"
No. 8
December 1997
"The Paper and Chambered Nautilus"
"Marine Biodiversity and Endemism in South Australia"
"Growth of Alexandrium minutum in the Port River"
"Diversity Within the Fish Order Scorpaeniformes"
"Fish Profile - The Dusky Morwong"
"Photo Index Update"
"Exploitations of Marine Flora and Fauna"
No. 9
December 1998
"Fish use of the Port River - Barker Inlet estuary"
"A Public Showcase for South Australian Marine Life"
"Sea Spiders"
"What’s Happening to Our Sharks?"
"Sea Grasses"
"Mediterranean Sea Worms"
"The Occurrence Of Oil And Chemical Spills In S. A’s Marine Waters"
"Determining the effects of an oil spill on fish communities in a mangrove-seagrass ecosystem in southern Australia"
"Marine Protected Areas"
"The Banded Morwong - Moving West?"
"Research and Education on Whales and Dolphins at the South Australian Museum"
"Script on the Preservation of Mangroves"
"Dragon Search Update"
"Photo Index Update"
No. 10
December 1999
"Spotted Handfish"
"A Stingaree Tale"
"The Port River Dolphins and their Environment"
"Stromatolites"
"Beachwatch"
"The Impact Of Bottom Trawling On The Benthic MacroinfaunaOf Gulf St Vincent, South Australia"
"The Effects of Trawling In South Australia’s Gulfs"
"MLSSA Photo Index Update"
"Marine Fossils in the Flinders Ranges"
"Eco-Logical Alter-Natives for Adelaide Coastal Plantings"
"From SA to SA Or Does that mean I’ve been going around in circles?"
No. 11
December 2000
"South Australian Dragon Search Project: Preliminary Bioregional Summary of Sighting Data April 1996 - August 2000"
"Inquiries Into The State Of The Marine Environment"
"The Amazing Giant Cuttle (Sepia apama)"
"A Footbridge Too Far"
"Fish Spine Injury and Envenomation"
"Journal Index"
"MLSSA Photo Index Update"
No. 12
December 2001
"Entangled Southern Right whale dies in Head of Bight whale sanctuary"
"Nicolas Baudin’s Scientific Expedition To The Terres Australes"
"Rapid Bay Jetty"
"A Whale by Any Other Name...."