Marine Life Society of South Australia Inc.
2004 Journal
TROY M. JANTZEN* AND JON N. HAVENHAND#
School of Biological Sciences, Flinders University, GPO Box 2100, Adelaide, South Australia, 5001
Received 15 February 2002; accepted 31 January 2003.
Reference: Biol. Bul. 204:290 - 304 (June 2003)
C 2003 Marine Biological Laboratory
* To whom correspondence should be addressed. E-mail: Troy.Jantzen@ bms.com
#
Current address: Tjärnö Marine Biological Laboratory, Göteborg University, 452 96 Strömstad, Sweden.Abstract.
Squids use a diverse range of body patterns for communication. Each pattern consists of a series of chromatic, postural, and locomotor components that are under neural control and can change within fractions of a second. Here we describe an ethogram of 48 body pattern components from in situ observations of reproductively active Sepioteuthis australis. In addition, we identify the total time and average duration that each component is shown, to a resolution of 1 s. Our results suggest that only a few components (e.g., "Golden epaulettes," "Stitchwork fins," and "Rigid arms") are temporally common, that is, shown for more than 80% of the time. In contrast to the component classification reported for other species of squid, for this species we suggest a classification system consisting of "short acute" (lasting for < 10 s); some of these same components were also classified as "medium acute" (11-60 s) or "chronic" (> 60 s). Several body patterning components were previously unreported, as were some of the combinations observed. The significance of these patterning components is discussed within the context of the associated behaviors of the squid on the spawning grounds.Introduction
Cephalopod behavior involves elaborate displays characterized by many visually apparent body patterns (Moynihan and Rodaniche, 1982; Hanlon, 1988; Hanlon et al., 1994, 1999; Hanlon and Messenger, 1996). In squid, each body pattern is a combination of postural, locomotor, or chromatic components that together constitute the total appearance of the animal (Packard and Sanders, 1969, 1971; Packard and Hochberg, 1977; Hanlon, 1982; Roper and Hochberg, 1988; Hanlon et al., 1994, 1999; Hanlon and Messenger, 1996). Of the three types, the most conspicuous and diverse to the human observer are the chromatic components. Chromatic changes result from neuromuscular expansion or retraction of pigmented chromatophore organs in the dermis of the skin (Boycott, 1953; Hanlon, 1982; Packard, 1982, Messenger, 2001). Dark chromatic components arise from the expansion of chromatophores, whereas light chromatic components are the result of retraction of chromatophores to reveal the translucent skin or iridescent reflecting cells (Hanlon, 1982; Hanlon and Messenger, 1996; Messenger, 2001). Postural components denote the postures of arms, tentacles, head, eyes, mantle, and fins; and loco-motor components describe movement of the whole animal (Hanlon and Messenger, 1996). Squid display multiple combinations of each component type that may last for several seconds ("acute patterns" sensu Packard and Hochberg, 1977; Hanlon and Messenger, 1996), or minutes to hours ("chronic patterns" sensu Hanlon and Messenger, 1996). Unfortunately, the classification of squid body patterns is not a simple task. Each chromatic component may be displayed alone, or combined with other components (simultaneously or in sequence) to generate the overall skin pattern of an individual (Hanlon et al., 1994, 1999; Mather and Mather, 1994). Thus, to analyze the nature of squid behavior, body components must first be cataloged, then each combination of components analyzed with respect to the circumstances in which each occurs (Mather and Mather 1994).
Body patterning and reproductive behavior are important tools in quantitative behavioral analysis, in the development of taxonomic identification keys, and for phylogenetic analyses of chromatic expression (Hanlon, 1988). Cephalopod body patterns have been studied for many decades, and the suite of body pattern components (ethogram) found in squids has been described for species such as Sepioteuthis sepioidea (Moynihan and Rodaniche, 1982), Loligo vulgaris reynaudii (Hanlon et al., 1994), Loligo pealeii (Hanlon et al., 1999), and several others (table 3.2 in Hanlon and Messenger, 1996).
The commercially important squid Sepioteuthis australis spawns throughout the year (Peel, 2000, 2001), with numbers of spawning adults in shallow coastal areas known to increase during warmer months. Current behavioral research on this species is limited to two preliminary observations of spawning activity, neither of which describes the repertoire of body patterns (Larcombe and Russell, 1971; Jantzen and Havenhand, 2003). In this study, we document and identify 48 components characteristic of S. australis reproductive behavior and describe the behaviors associated with each component. In addition, we analyze the occurrence, duration, and frequency of each component.
Materials and Methods
Mating behavior of Sepioteuthis australis in the field was observed by scuba and directly from the surface. The presence of divers close to focal animals (< 30 cm) caused no apparent alteration in squid behavior (compared with individuals observed from afar). Observations were therefore routinely made at a distance of less than 2 m.
Reproductively active individuals of S. australis were observed by scuba diving and snorkeling during daylight hours on spawning grounds between Marino and Hallet Cove, South Australia (138°29' E, 38°02' S; see Jantzen and Havenhand, 2003, for a description of the site) over three consecutive spawning seasons (September to March) between September 1999 and February 2002. Digital video data were obtained from 50 dives over an 18-week period from November 2000 to March 2001.
Ethogram of reproductive body patterns
Reproductive behavior was recorded with a Sony TV120 digital video camera (Sony Corporation, New York) in an Amphibico (Amphibico, Quebec, Canada) housing. Sampling protocols followed those described by Martin and Bateson (1993), which included small amounts of ad libitum sampling of specific behaviors. However, the primary sampling method was focal-animal sampling, which involved recording all interactions and behaviors of haphazardly chosen paired individuals for up to 1.5 h. Because males and females are close together when paired, analysis of the focal sampling of one individual provided information on the corresponding behavior of its mate.
Images of behavior were analyzed frame by frame (25 frames • s-1) on a high-resolution large-screen color monitor. Each video was analyzed three times to identify postural, locomotor, or chromatic components. All components were assigned a name based either on established component nomenclature for other cephalopod species (Corner and Moore, 1980; Hanlon, 1982, 1988; Moynihan and Rodaniche, 1982; Moynihan, 1985; Yang et al., 1986; Porteiro et al., 1990; Hanlon et al., 1994, 1999; Jantzen and Haven-hand, 2003); or, where a new chromatic component was identified, on guidelines proposed by Hanlon and Messenger (1996) in which transverse components are called "bands" or "bars," and lines running longitudinal to the body axis called "stripes" or "streaks." In accordance with convention, only the first letter of each component name was capitalized (Hanlon and Wolterding, 1989).
Still images of selected chromatic and postural components were obtained from digital video using iMovie2 software (Apple Computer, Cupertino, CA). Each chromatic component was then illustrated using CorelDraw 3.0 (Corel Corporation, Ontario, Canada) from a lateral perspective (observations were mostly lateral to the focal animal).
Total component duration
Each chromatic, postural, and locomotor component was scored individually (present, absent, data missing) for each squid at a 1-s-interval scale for the duration of the video, so that the proportion of time each component was displayed could be assessed. Missing values were classed as any points when the body of the squid (or part thereof) was not in the field of view (with the exception of "Oviposition," during which females were obscured by seagrass). All missing data (zeros) were eliminated from the analysis. Because video analysis frequently took several hours, the first two squid pairs analyzed were re-analyzed at the end of the analysis session and compared with the original analysis to ensure consistency in interpretation and data recording. Because significant experimental error in the perception of components may occur between observers in behavioral investigations (Drummond, 1981), all analyses were conducted by the same person (TMJ).
The behavior of 22 focal animals (11 female, 11 male) was recorded for a continuous duration of up to 61 min per pair. The total occurrence of each component (in seconds) was determined with respect to the total number of valid data points (missing values excluded) for each animal. Mean total duration of each component as a proportion of total time observed was calculated from data for individuals that showed the component being analyzed (data for animals not showing a component were omitted). Means generated from fewer than three replicate animals were not included in further analysis. Means and 95% confidence intervals were generated for each component.
Pairwise Student’s t tests were conducted on the duration of each component shown by males and females within replicate pairs. This procedure tested the hypothesis that there was no significant difference in mean component duration between sexes. Because this involved multiple unplanned tests with consequent information of Type I errors, a sequential Bonferroni test was conducted on the results from the t test to determine significance against the table-wide α-level (Rice, 1989).
Average component duration
The average duration of each component was calculated from the "uninterrupted" data (i.e., excluding all component observations that began or ended due to the beginning or end of video recording, or were interrupted by missing values). Average component duration was calculated for each replicate individual, and a mean value was generated for all females or males. Not all components were shown by each individual; therefore, means from fewer than three replicates were, as before, discarded from the analysis.
As with the data for total component duration, pairwise t tests were conducted on the average duration of each component shown by males versus females within pairs.

Results
Several hundred reproductively active individuals of Sepioteuthis australis were observed in more than 75 h underwater. About 10 h of focal-sampling video observations of behavior were recorded. The number of reproductively active individuals ranged from 2 (a single spawning pair) to more than 45 per dive. From these data we identified 48 separate body components: 17 chromatic (Table 1, Fig. 1), 16 postural (Table 1, Fig. 2), and 15 locomotor (Table 1). Mating, egg deposition, and agonistic contests were all seen on the spawning grounds in the same manner as described previously by Jantzen and Havenhand (2003).
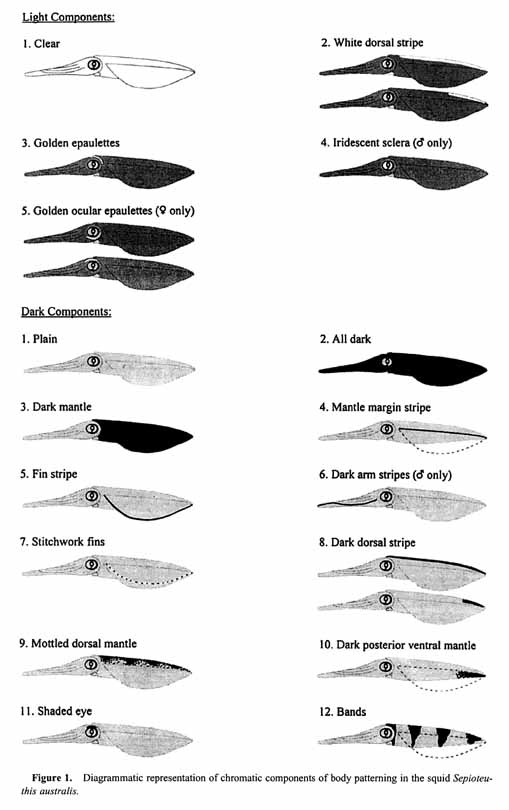
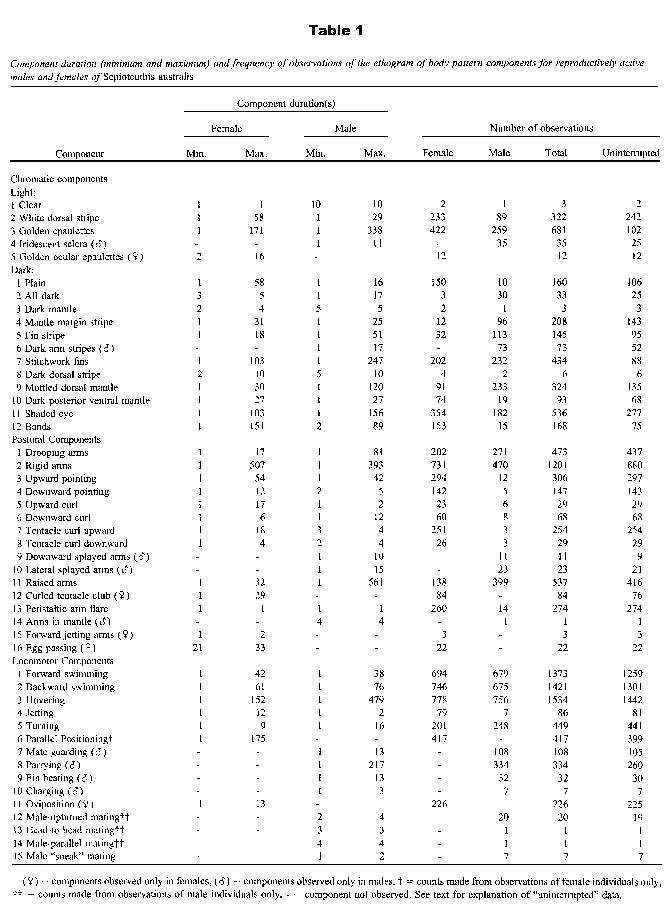
Figure 1.
Diagrammatic representation of chromatic components of body patterning in the squid Sepioteuthis australis.Ethogram of reproductive body patterns
Components that were previously undescribed or differ from those in the literature (Comer and Moore, 1980; Hanlon, 1982, 1988; Moynihan and Rodaniche, 1982; Moynihan, 1985; Yang et al., 1986; Porteiro et al., 1990; Hanlon et al., 1994, 1997, 1999; Jantzen and Havenhand, 2003) are described below. Where components do not differ from existing definitions, the relevant literature has been cited. Unless stated otherwise, components were observed in both sexes, lateral to the focal animal.
Results for individual components are described below, summarized in Table 1, and illustrated in Figures 1 and 2. Combinations of components for typical behaviors are presented in Figure 3. Photographs of common combinations of chromatic and postural components are shown in Figure 4.
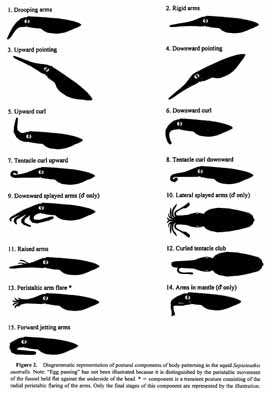
Figure 2.
Diagrammatic representation of postural components of body patterning in the squid Sepioteuthis austmlis. Note: "Egg passing" has not been illustrated because it is distinguished by the peristaltic movement of the funnel held flat against the underside of the head. * = component is a transient posture consisting of the radial peristaltic flaring of the arms. Only the final stages of this component are represented by the illustration.1. Light chromatic components
Clear (Hanlon, 1988; Yang et al., 1986; Porteiro et al., 1990; Hanlon et al., 1994, 1999; Cornwell et al., 1997) was rarely observed (three observations from focal sampling; Table 1) and on each occasion was shown alone as a body pattern. White dorsal stripe was a lightened stripe in the center of the dorsal mantle and varied in intensity; it either extended along the entire longitudinal length of the dorsal surface or was restricted to the posterior region. This component often began at the posterior region and extended to cover the entire longitudinal dorsal mantle. Without exception, females showed "White dorsal stripe" in combination with "Male-upturned mating" (see below and Fig. 3). Golden epaulettes was a lightened band at the most anterior point of the dorsal mantle that occurred as a result of the contraction of chromatophores (referred to as "Dorsal mantle collar iridophores" by Hanlon, 1988; Hanlon et al., 1994, 1999). This was the most prevalent chromatic component (Table 1) and was shown seemingly indiscriminately with almost all other components. Iridescent sclera occurred when the iris of the eye became iridescent, it was observed only in agonistic contests between fighting males (Fig. 3), usually in conjunction with "All dark" (see below). Golden ocular epaulettes (described above the eye only as "Light eyebrows" or "Glittering brows" in S. sepioidea; Moynihan and Rodaniche, 1982; Moynihan 1985) were observed as a lightened region above and/or below the eye. This component was observed only in females (Table 1) and differs from "Iridescent sclera" in that the area adjacent to the eye becomes iridescent.
Figure 3. Components consistently identified in agonistic encounters, egg preparation, egg deposition, paired mating, and extra-pair copulations in Sepioteuthis australis. Numbers correspond to component numbers in Table 1; letters correspond to component types from Table 1; Cl = Light chromatic, Cd = Dark chromatic, P = Postural, L = Locomotor, ♀ = component shown by females; ♂ = component shown by males.
2. Dark chromatic components
We describe the manner in which dark chromatic components influence overall appearance without reference to the individual contributions of yellow, orange, red, brown, or black chromatophores (Hanlon and Messenger, 1996), which we were not able to analyze.
Plain was shown as a medium brown coloration over the entire body (referred to as "Basic" by Moynihan and Rodaniche, 1982; Moynihan, 1985). This component was more common in females (Table 1) and was especially seen in females that were "Egg passing" (see below). It was also shown by unpaired males attempting to "Male ‘sneak’ mate" (see below), and by courting pairs. All Dark (Hanlon, 1988; Hanlon et al., 1994, 1999; Cornwell et al., 1997) was shown by fighting males that were "Fin beating" (see below and Fig. 3). A variation of the "All dark" component was Dark mantle, in which only the mantle was darkened while the head and arms remained "Plain." This component was rare (Table 1), and the behavior associated with it remains unclear.
Although also shown independently, the following four components were all shown together by paired males in the seconds immediately prior to "Male-upturned mating" (see below and Fig. 3). Mantle margin stripe (referred to as "Fin stripe" by Moynihan and Rodaniche; 1982, but here we follow the nomenclature of Hanlon et al., 1999) was a darkened stripe running along the fin insertion the entire length of the mantle. Fin stripe (Hanlon et al., 1994, 1999) occurred as a darkening of the edge of the fin. Dark arm stripes (referred to as "Dark third arms" by Porteiro et al., 1990, and "Arm stripe (first or third)" by Hanlon et al., 1994, but here we follow the nomenclature of Hanlon et al., 1999) involved the darkening of the most lateral arms and was shown exclusively by males (Table 1). Shaded eye was expressed as a transverse pigmented "bar" spanning the two eyes (as described in other squid species by Hanlon, 1988; Hanlon et al., 1994, 1999) or as a shading immediately over the eye only. This component was common to both sexes (Table 1).
Stitchwork fins (Hanlon, 1982) comprised alternating black and white dashes that traversed the fins 2-3 cm from the edge. This component was one of the most common chromatic components shown by paired individuals (Table 1). Dark dorsal stripe was a darkened stripe along the center of the dorsal mantle. This component varied in length, either extending along the entire dorsal surface of the mantle or being shown only in the posterior region. We have few observations of this component (Table 1) and the behavior associated with it remains unclear. Mottled dorsal mantle was an irregular pattern of darkened spots on the dorsal mantle surface grouped together to form a mottle, occasionally extending to include the fins. Paired males showed this component in nonphysical agonistic encounters such as "Parrying" (see below). This component may be cryptic, as the irregularity of the pattern may disrupt the body shape when viewed from above (by predators). Dark posterior ventral mantle was an irregular darkening of the posterior ventral mantle (underneath the fins) up to a third of the length of the mantle in both sexes. This was more frequently seen in females (Table 1), but we remain uncertain of its behavioral significance.
Bands is also referred to as "Bars" by Moynihan and Rodaniche (1982) and Moynihan (1985) and as "Ring" by Hanlon (1982), but we follow the nomenclature of Hanlon et al. (1994, 1999) and Cornwell et al. (1997). This component was observed on all occasions as four transverse bands around the dorsal and ventral mantle. A single longitudinal stripe on the ventral mantle, similar to "Ventral mantle stripe" in S. sepioidea (Moynihan and Rodaniche, 1982), was also seen in conjunction with this pattern: because it was never observed independently, it is considered to be an integral part of the "Bands" component in S. anstralis. As in other loliginid squid, this component possibly aids in crypsis and was common in females of lone spawning pairs.
3. Postural components
Drooping arms occurred when the tentacles and arms of the individual were not in a "defined" posture and appeared limp. This component was reported by Hanlon et al. (1999) to occur in Loligo pealei individuals that were swimming, but we observed this behavior primarily in "Hovering" (see below) individuals of S. australis as well as in swimming ones. Rigid arms occurred when all arms and tentacles were held together immediately in front of the animal and was the most common arm position of paired animals (Table 1). For consistency in the analysis, the transition from "Rigid arms" to "Drooping arms" was defined as the moment when the average angle of the arms and tentacles exceeded 45° downward from horizontal. Upward pointing and Downward pointing were scored when the orientation of the whole body exceeded 45° from horizontal in the respective direction. Both of these components were far more prevalent in females (Table 1). "Upward pointing" was more often seen in individuals close to the substrate while "Hovering," but "Downward pointing" was frequently observed in females that were "Forward swimming" (see below) prior to deposition of an egg capsule (Fig. 3). Upward curl and Downward curl were identical in posture to the descriptions given by Moynihan and Rodaniche (1982) for S. sepioidea. Tentacle curl upward was the simultaneous rolling back of the tip of the tentacles dorsally along the arms to form a tight circle with the suckers on the club facing out. This component always occurred in the seconds prior to "Oviposition" by the female (Fig. 3) and was a reliable predictor of egg deposition behavior. Tentacle curl downward was the rolling back of the tentacle tips downward, along the ventral surface of the arms with the suckers facing inward. This component was rare (Table 1), and the association with specific behaviors remains unclear.
Downward splayed arms (Fig. 4C) was observed only in males (Table 1). The arms and tentacles curled around posteroventrally from the "Rigid arms" posture so that each tentacle was offset from the next. The significance of this component remains unknown. Lateral splayed arms (as "Splayed arms" in Hanlon et al., 1999) was recorded in the latter stages of a fight between males, usually coinciding with "Fin beating" (see below and Fig. 3). A series of these components in rapid succession (< 1 s each) was also shown by several paired males poised above the female immediately prior to rotating to the upside-down position for "Male-upturned mating" (see below and Fig. 3). It is as yet unclear whether the duration of this component is significant in these different behaviors. Raised arms differed from the observations of this component in L. pealeii (Hanlon et al., 1999) in that only the tips of the fourth pair of arms were raised, rather than the entire first pair of arms. This component was shown primarily by paired males (Table 1). As in L. vulgaris reynaudii (Hanlon et al., 1994), this is thought to be an intraspecific agonistic signal because we consistently saw it in males that were "Parrying" (see below and Fig. 3).
Curled tentacle club was observed only in a single female; however, Jantzen and Havenhand (2003) previously identified this component in males of S. australis (as "Club display"). Peristaltic arm flare was a rapid component lasting for ≤ 1 s (Table 1) and involved the peristaltic flaring of the tentacles and arms from the base to the tips as the animal simultaneously pulsed a jet of water across the arms (referred to as "Puffing" by Comer and Moore, 1980, and as "Cleaning maneuver" by Packard and Sanders, 1971). It appeared to be associated with the removal of excess matter from amongst the arms, and was most prevalent in females after "Oviposition" (Fig. 3). Arms in mantle occurred when all of the arms were positioned inside the ventral mantle while the tentacles adopted the "Drooping arms" posture. Forward jetting arms occurred when the arms and tentacles were directed posterioventrally coinciding with the rapid forward motion of the animal as it "Jetted" (see below) forward. Egg passing identifies a behavior in which females transferred eggs from the oviduct through the funnel into the arms. "Egg passing" was synonymous with the funnel being positioned flat against the underside of the head, with the funnel aperture opening into the bases of the arms. During this behavior, the ventral region of the arm bases enlarged as the eggs and egg capsule material were passed into the arms.
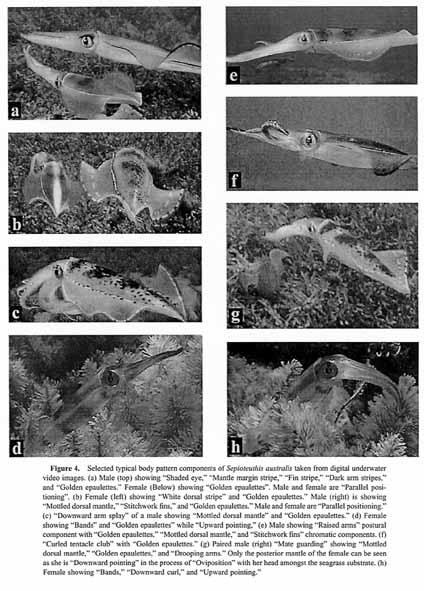
Figure 4.
Selected typical body pattern components of Sepioteuthis australis taken from digital underwater video images, (a) Male (top) showing "Shaded eye," "Mantle margin stripe," "Fin stripe," "Dark arm stripes," and "Golden epaulettes." Female (Below) showing "Golden epaulettes". Male and female are "Parallel positioning". (b) Female (left) showing "White dorsal stripe" and "Golden epaulettes." Male (right) is showing "Mottled dorsal mantle," "Stitchwork fins," and "Golden epaulettes." Male and female are "Parallel positioning." (c) "Downward arm splay" of a male showing "Mottled dorsal mantle" and "Golden epaulettes." (d) Female showing "Bands" and "Golden epaulettes" while "Upward pointing," (e) Male showing "Raised arms" postural component with "Golden epaulettes," "Mottled dorsal mantle," and "Stitchwork fins" chromatic components, (f) "Curled tentacle club" with "Golden epaulettes." (g) Paired male (right) "Mate guarding" showing "Mottled dorsal mantle," "Golden epaulettes," and "Drooping arms." Only the posterior mantle of the female can be seen as she is "Downward pointing" in the process of Oviposition" with her head amongst the seagrass substrate, (h) Female showing "Bands," "Downward curl," and "Upward pointing."4. Locomotor components
Forward swimming, Backward swimming, Hovering, Jetting (Moynihan and Rodaniche, 1982; Hanlon et al., 1994, 1999), and Turning are self-explanatory. For consistency of analysis, Parallel positioning was defined here as paired squid aligned laterally and facing in the same direction, where any part of the bodies were closer than the paired male’s body length. The high frequency of this component (Table 1) is indicative of the relative closeness of reproductively active pairs. Mate guarding is common in loliginid squids (Hanlon and Messenger, 1996) and was defined here as being a consort male within one body length of the female that was in the process of "Oviposition" (Fig. 3). Paired males frequently showed "Mottled dorsal mantle" while "Mate guarding."
The following three components are varying levels of agonistic encounters between males (Fig. 3). Parrying was the active positioning of the paired male between the female and an intruder male, within one body length of the intruder. No physical contact occurred between the intruder and paired male at this time. Fin beating (Hanlon et al., 1994, 1999; Jantzen and Havenhand, 2003) was the escalation of an agonistic contest into physical contact. The two males collided, beating their mantle and fins together. "Fin beating" consistently occurred with "Dark mantle" and "Iridescent sclera." Charging was defined as a rapid movement of a paired male toward a rival male, striking the intruder with the tentacles and radially flaring the arms. This is an intense agonistic encounter by a paired male and appeared similar to that described for L. pealeii (King et al., 1999) and Loligo forbesi (Porteiro et al., 1990).
Oviposition (Hanlon et al., 1999; Jantzen and Haven-hand, 2003) denotes the process of egg deposition by a female (Fig. 3). Male-upturned mating (Jantzen and Havenhand, 2003) occurred when the male rotated laterally to an upside-down position above the female to mate. Head-to-head mating (Hanlon et al., 1999) occurred when the mating pair approached head-on and intertwined arms to mate. Male-parallel mating (Hanlon et al., 1994, 1999) occurred when the paired male approached the female from below and attempted to mate facing the same direction. "Plain" was the only chromatic component shown consistently throughout these prior two mating types (Fig. 3). Male "sneak" mating (Hanlon, 1994; Jantzen and Havenhand, 2003) was defined as any mating attempt by an unpaired male with a paired female. Sneaker males also commonly showed the "Plain" chromatic components, or (infrequently) "Dark mantle" and "White dorsal stripe" (Fig. 3).
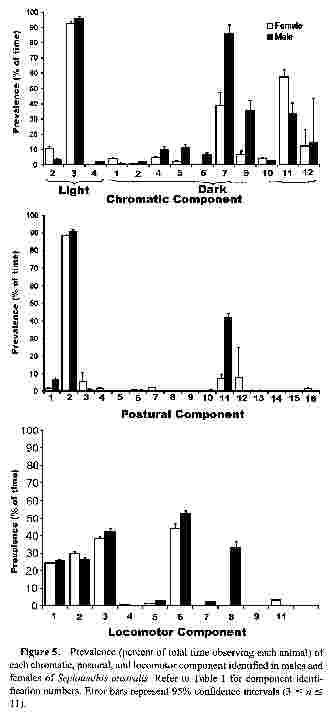
Figure 5.
Prevalence (percent of total time observing each animal) of each chromatic, postural, and locomotor component identified in males and females of Sepioteuthis australis. Refer to Table 1 for component identification numbers. Error bars represent 95% confidence intervals (3 ≤ n ≤ 11).Total component duration
The most prevalent chromatic component for both sexes was "Golden epaulettes" ("Chromatic Light 3" in Fig. 5), shown for more than 90% of the time. "Stitchwork fins" was also common in males (85% of the time; "Chromatic Dark 7" in Fig. 5), but was less prevalent in females. Other components prevalent in males were "Mottled dorsal mantle" (30%; "Chromatic Dark 9" in Fig. 5), and "Shaded eye" (20%; "Chromatic Dark 11" in Fig. 5). In females, "Shaded eye" (42%) and "Stitchwork fins" (22%) were also prevalent ("Chromatic Dark 11 and Chromatic Dark 7" in Fig. 5).
"Rigid arms" was the only postural component commonly shown by both sexes and was observed about 90% of the time ("Postural 2" in Fig. 5). "Raised arms" was also common in males (42%; "Postural 11" in Fig. 5).
The prevalences of the locomotor components "Forward swimming," "Backward swimming," and "Hovering" were much the same for both sexes ("Locomotor 1, 2, and 3" in Fig. 5). "Parallel positioning" occured ~ 50% of the time (both sexes; "Locomotor 6" in Fig. 5), and males spent 34% of the time "Parrying rival" males ("Locomotor 8" in Fig. 5).
Pairwise t tests of the differences in total component durations between males and females showed that only the durations of "Stitchwork fins" were significantly different (sequential Bonferroni test, table-wide α = 0.05). Males showed this pattern for considerably longer periods (Fig. 5, Table 1).
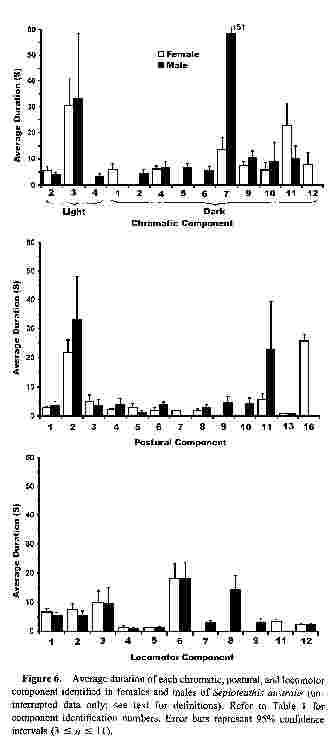
Figure 6.
Average duration of each chromatic, postural, and locomotor component identified in females and males of Sepioteuthis australis (uninterrupted data only; see text for definitions). Refer to Table 1 for component identification numbers. Error bars represent 95% confidence intervals (3 ≤ n ≤ 11).Average component duration
Components that were shown by both males and females had a similar average duration. Only "Stitchwork fins," "Shaded eye," and "Raised arms" showed a large difference in average duration between the sexes ("Chromatic Dark 7," "Chromatic Dark II," and "Postural 11" respectively; Fig. 6). However, variance was generally high, and pairwise t tests of the differences in average component durations between males and females showed that only "Forward swimming" differed significantly (sequential Bonferroni test, table-wide α = 0.05).
Most (80%) of the components had an average duration less than 10 s (Fig. 7), 86% had durations less than 20 s, 95% were less than 30 s, and all were less than 60 s. Consequently, all of the components identified in this study can be considered acute (lasting for seconds or minutes; sensu Box 3.1, Hanlon and Messenger, 1996). Several of these same components may also be considered chronic (lasting minutes to hours; sensu Hanlon and Messenger, 1996) when the maximum duration of each component is also considered (Table 1).
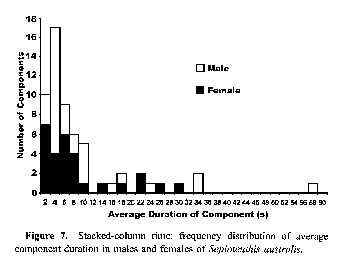
Figure
7. Stacked-column time: frequency distribution of average component duration in males and females of Sepioteuthis australis.Discussion
All of the components we identified in Sepioteuthis australis had a mean duration less than 60 s (Figs. 5 and 6) and should therefore be classified as "acute" (i.e., lasting for seconds or minutes, sensu Box 3.1, Hanlon and Messenger, 1996). However, because 80% of the components had a duration ≤ 10 s (Fig. 7), the classification may need to be refined to take account of varying levels of "acute" response. We propose a three-tier system consisting of short acute (≤ 10 s), medium acute (11-60 s), and chronic (> 60 s) component durations. Using this system, and treating the components shown by each sex separately, we classify 32% (25/77) of the components in S. australis as short acute, 43% (33/77) as both short acute and medium acute, and 25% (19/77) as short acute, medium acute, and chronic (Table 1). This system also permits identification of patterning differences that may be biologically meaningful. For example, components such as "Mottled dorsal mantle," "Drooping arms," and "Raised arms" all had acute durations (short and medium) in females of the species (Table 1), but these same components had chronic as well as acute (short and medium) durations in males. A thorough interpretation of these differences is beyond the scope of the present work; however, we believe that this additional distinction within "acute" will prove useful for interpreting the utilization of cephalopod component signals.
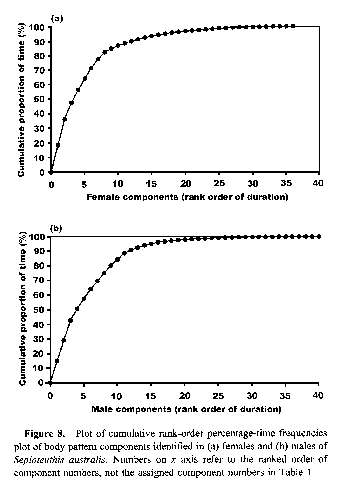
Figure 8.
Plot of cumulative rank-order percentage-time frequencies plot of body pattern components identified in (a) females and (b) males of Sepioteuthis australis. Numbers on x axis refer to the ranked order of component numbers, not the assigned component numbers in Table 1.The ethogram of reproductive body components presented in this study has both similarities to and differences from those described for other species (Hanlon, 1988; Yang et al., 1986; Porteiro et al., 1990; Hanlon et al., 1994,1999). For example, similarities are seen in the "Clear" chromatic component, which was previously identified as an acute component by Hanlon (1988), Yang et al. (1986), Porteiro et al. (1990), and Hanlon et al. (1994, 1999) and also found here to be a short acute component in S. australis. Likewise, "Mate guarding" has been identified as an acute component in Loligo pealeii (Hanlon et al., 1999) and was also defined as an acute (short and medium) component here for S. australis. In contrast, "Dark dorsal stripe" (Hanlon et al., 1994) has previously been identified only as a chronic component, but was identified in our study as a short acute component (Table 1, and see above).
The high number of short acute component durations we identified (Table 1, Fig. 6) indicates intense communication between reproductively active individuals and is probably important in competition for mates and in predator detection. Guilford and Dawkins (1991) have argued that short, intense animal signals (lasting only a few seconds) are more information-rich than longer, weaker signals. The corollary of the observed intensity of short acute component durations is that reproductively active individuals of S. australis showed few long (medium acute, or chronic) duration components (Fig. 6). It is possible that signals are communicated by relatively brief absences of these longer duration components (rather than by their chronic presence); however, their significance has yet to be determined.
"Golden epaulettes," "Stitchwork fins" (male only), and "Rigid arms" were all shown for over 80% of the total observation time (see Fig. 5), and these three same components also had the longest average durations (see Fig. 6). Because long component durations were more strongly affected than short durations by our procedure of counting only uninterrupted recordings, more than 80% of the data for "Golden epaulettes" and "Stitchwork fins" was omitted due to interruptions. Consequently, average durations in Figure 6 are probably a marked underestimate of the true durations for these components.
If the catalog of behaviors observed for a species is representative of the entire behavioral repertoire, then a plot of the cumulative rank-order percentage-time frequencies of the behaviors should show a marked asymptote (Lehner, 1979). Relevant plots for our data show an asymptote for both females and males after 30 components (Fig. 8). We identified 38 components for females and 44 for males (Table 1). These numbers exceed the point at which an asymptote is reached in each data set and therefore suggest that the data reported here are indeed a representative catalog of the component repertoire for the reproductive behavior of this species (Lehner, 1979).
The number of chromatic components we identified (n = 17) is less than that described for S. lessoniana (23; Moynihan and Rodaniche, 1982; Moynihan, 1985; Hanlon and Messenger, 1996) and S. sepioidea (23: Moynihan and Rodaniche, 1982; Hanlon and Messenger, 1996). However, our data for S. australis (17 chromatic components, Table 1) relate only to reproductive behavior. Of the 23 chromatic components reported by Moynihan and Rodaniche (1982) for S. sepioidea, 17 were observed in reproductively active individuals (i.e., 75% of the total repertoire). In total, 23 chromatic components were also identified in S. lessoniana (Hanlon and Messenger, 1996), although the number of these components specific to reproductive behavior is not known. We were unable to identify body pattern components associated with nonreproductive behavior, and therefore we do not know the full component complement for S. australis. Nonetheless, the full repertoire of chromatic components in cephalopods is thought to be correlated with habitat complexity (table 3.9 in Hanlon and Messenger, 1996). Given that S. australis lives in a structurally less complex habitat than S. sepioidea (seagrass versus coral reefs), it seems likely that the ethogram of reproductive components identified here is a large proportion of the entire ethogram for this species.
As in this current study, the body patterning behavior of L pealeii (Hanlon et al., 1999), and L. vulgaris reynaudii (Hanlon et al., 1994) was described primarily from individuals on spawning grounds. It is therefore useful to compare the ethograms of these two Loligo species with that of S. australis. For L. pealeii, 30 chromatic, 5 postural, and 12 locomotor components (plus a further 4 polarized components) were identified (Hanlon et al., 1994); for L. vulgaris reynaudii, the numbers were 23 chromatic, 4 postural, and 9 locomotor components (Hanlon et al., 1994). It seems that these two Loligo species have a larger repertoire of chromatic components than S. australis has, but the repertoire of postural and locomotor components appears considerably larger for S. australis. Differences in chromatic component numbers can probably be attributed to differences in habitat structural complexity (table 3.9 in Hanlon and Messenger, 1996), but the significance of differences in postural and locomotor component numbers between species is unclear.
When comparing the ethograms of L. pealeii (Hanlon et al., 1999), L. vulgaris reynaudii (Hanlon et al., 1994), and S. australis, many of the component patterns appear similar, but the comparative frequency that each component is shown varies markedly. The prevalence of some components is quite different between all three species. For example, "All dark" was the most prevalent dark chromatic component shown by L. pealeii (Hanlon et al., 1999), it was shown moderately by L. vulgaris reynaudii (Hanlon et al., 1994), and it was one of the least common dark chromatic components shown by S. australis (Table 1). Alternatively, the display frequency of some components shows signs of differentiation between the two genera. For example, "Clear" was frequently shown by both L. pealeii (Hanlon et al., 1999) and L. vulgaris reynaudii (Hanlon et al., 1994), but was the least shown light chromatic component for S. australis. Body pattern components of behavior are thought to be useful in taxonomic distinction between squid species (Hanlon, 1988; Roper and Hochberg, 1988), and discussions such as the above demonstrate some of the comparisons that can be made.
As well as comparisons of ethograms between species, comparisons between members of a species that are and are not reproductively active could provide an important insight into the visual vocabulary associated with spawning. Anecdotal observations of non-reproductively active (feeding) squids showed that the most common postural component (> 50%) was "Downward pointing" (perhaps as an aid to prey acquisition). We observed the "Downward pointing" posture in reproductively active individuals only in females and only for 2% of the total time (Fig. 5); it generally occurred when the female was seeking out the egg cluster on the substratum prior to egg deposition. Consequently, while our analysis suggests that the ethogram of reproductive components presented here adequately describes the reproductive body patterns of S. australis, it seems equally clear that this ethogram does not describe the suite of body patterns exhibited by non-reproductively active individuals.
In a previous study, we reported that "Curled tentacle club" (as "Club display"; Jantzen and Havenhand, 2003) was shown by a male to the female as a signal of intention to mate. It is now clear that this was incorrect. Firstly, all 84 incidences of "Curled tentacle club" in this study were in females (Table 1); secondly, the frequency of this component was not related to copulation frequency.
Each of the four mating types reported here for S. australis ("Male-upturned mating," "Head-to-head mating," "Male-parallel mating," and "Male "sneak" mating") had a maximum duration of 4 s (Table 1). It cannot be ruled out that "Male-upturned mating" and "Head-to-head mating" are derivatives of the same mating type, as it is likely that the site of spermatophore deposition is in the arms for both these mating types. However, because the deposition site for "Head-to-head mating" in S. australis could not be identified here, we believe these two mating types should be considered as separate behaviors. Interestingly, "Male-upturned mating" was the most common mating type observed here (20 out of 29 matings; Table 1). This mating type has been observed in only one other species of squid (S. lessoniana) and only in the laboratory (Boal and Gonzalez, 1998). Those authors report a mating duration similar to that reported here (3 s; Table 1, Fig. 6). Durations of other mating types seen here were also comparable to those observed in other squids (see Segawa et al., 1993; table 6.2 in Hanlon and Messenger, 1996). This suggests that the durations of these behaviors in different species may have evolved in response to similar selective pressures.
Identifying the selective benefits of a given patterning component or behavior is difficult. For example, immediately prior to mating, paired males of S. australis always showed "Mantle margin stripe," "Fin stripe," "Dark arm stripes," and "Shaded eye" chromatic components. This proved to be a reliable predictor of a "Male-upturned mating" attempt (Fig. 3). However in Loligo vulgaris reynaudii, "Fin stripe," Mantle margin stripe," and "Arm stripes" are all intraspecific signals in agonistic contests between males (Hanlon et al., 1994). Boal (1997) suggested that the mating displays of male cuttlefish function as agonistic signals to other males rather than courting signals to females. Whether these components portray different messages in these two species, or are used agonistically towards other males during mating in S. australis is unclear.
"Lateral splayed arms" was shown by males of S. australis in association with "Male-upturned mating" as well as in agonistic encounters with rival males. This signal has been identified as exclusively agonistic in L. vulgaris reynaudii (Hanlon et al., 1994), L. plei (DiMarco and Hanlon, 1997), and L. pealeii (Hanlon et al., 1999), although in S. sepioidea it is reportedly both agonistic (Moynihan, 1985; Hanlon and Messenger, 1996) and associated with mating (Moynihan and Rodaniche, 1982). Again, it is possible that this component portrays different messages in different species; however, it is also possible that the duration of this component differs subtly between mating and agonistic behaviors. We could not distinguish any consistent differences from our analyses, so this possibility remains to be tested.
In contrast to reports in the literature, we found that mating types were not used consistently within males. Indeed, some males copulated with the same female in all three paired mating positions ("Male-upturned mating," "Head-to-head mating," and "Male-parallel mating"). Previously, no more than three mating positions had been reported within a single squid species (table 6.2 in Hanlon and Messenger, 1996). Whether paired males of S. australis show (or have shown) "Sneaker mating" at other times is not known; however, this evidence suggests that S. australis may exhibit a greater diversity in mating positions (three, plus "Sneaker mating") than other squids.
We identified three types of agonistic encounters between rival males: "Parrying," "Fin beating," and "Charging" (Fig. 3). While "Parrying" represented a nonphysical intraspecific encounter, "Fin beating" and "Charging" involved physical contact between rival males. "Parrying" (34% of time. Fig. 5; 334 observations, Table 1) was far more prevalent than "Fin beating" (1% of time, Fig. 5; 32 observations. Table 1) and "Charging" (7 observations. Table 1). In addition, "Parrying" had a longer average duration (15s, Fig. 6) than "Fin beating" (4 s, Fig. 6). This behavior is similar to the fighting tactics of L. plei in which physical agonistic contests between rival males are shorter than nonphysical contests (DiMarco and Hanlon, 1997).
In a wider context, chromatic components of behavior are useful in taxonomic distinction of squid species (Hanlon, 1988; Roper and Hochberg, 1988). Comparative allozyme analysis of S. australis has identified a discrete genetic divergence between the New Zealand and Australian populations (Triantafillos and Adams, 2001). It is possible that these populations have also evolved a different repertoire of reproductive components. The only report of the reproductive behavior of S. australis from New Zealand (Larcombe and Russell, 1971) describes body components generally consistent with "Clear," "Hovering," "Parallel positioning," "Rigid arms," "Oviposition," "Mate guarding," "Downward pointing," "Plain," "Peristaltic arm flare," "Charging," and "Parrying"; unfortunately, their descriptions lack sufficient detail to be directly compared with our observations. Further comparative research on the repertoire, frequency, and duration of reproductive components between these two populations would be valuable. Not only would this aid our understanding of the evolution of behavioral mechanisms or reproductive isolation in cephalopods, but it would also cast considerable light on the behavioral differences between populations of the species observed here.
Acknowledgments
We are grateful to J. Doube, M. Gemer, A. Hirst, D. Keuskamp, L. Kupriyanova, B. Lock, A. Mack, G. Mack, M. Naud, and V. Weisbecker for helping with underwater activities, to C. Turner for assistance with data recording and entry, and especially to R. Hanlon for assistance with the nomenclature of components and important comments on this manuscript. We also thank P. Fairweather, L. van Camp, and an anonymous reviewer for valuable comments and suggestions on this manuscript. Video equipment was obtained from Sea Optics Australia Pty. Ltd. This work was supported by an Australian Postgraduate Award Scholarship (to TMJ), and a grant from the Australian Research Council (to JNH).
Literature Cited
Boal, J. G. 1997. Female choice of males in cuttlefish (Mollusca: Cephalopoda). Behaviour 134: 975-988.
Boal, J. G., and S. A. Gonzalez. 1998. Social behaviour of individual oval squids (Cephalopoda, Teuthoidea, Loliginidae, Sepioteuthis lessoniand) within a captive school. Ethology 104: 161-178.
Boycott, B. B. 1953. The chromatophore system of cephalopods. Proc. Linn. Soc. Lond. 164: 235-240.
Corner, B. D., and H. T. Moore. 1980. Field observations on the reproductive behavior of Sepia latimanus. Micronesia 16: 235-260.
Cornwell, C. J., J. B. Messenger, and R. T. Hanlon. 1997. Chromatophores and body patterning in the squid Alloteuthis subulata. J. Mar. Biol. Assoc. UK 77: 1243-1246.
DiMarco, F. P., and R. T. Hanlon. 1997. Agonistic behavior in the squid Loligo plei (Loliginidae, Teuthoidea): fighting tactics and the effects of size and resource value. Ethology 103: 89-108.
Drummond, H. 1981. The nature and description of behavior patterns. Pp. 1-33 in Perspectives in Ethology, Volume 4, Advantages of Diversity, P. P. G. Bateson and P. H. Klopfer, eds. Plenum Press, New York.
Guilford, T., and M. S. Dawkins. 1991. Receiver psychology and the evolution of animal signals. Anim. Behav. 42: 1-14.
Hanlon, R. T. 1982. The functional organization of chromatophores and iridescent cells in the body patterning of Loligo plei (Cephalopoda: Myopsida). Malacologia 23: 89-119.
Hanlon, R. T. 1988. Behavioral and body patterning characters useful in taxonomy and field identification of cephalopods. Malacologia 29: 247-264.
Hanlon, R. T., and J. B. Messenger. 1996. Cephalopod Behaviour. Cambridge University Press, Cambridge.
Hanlon, R. T., and M. R. Wolterding. 1989. Behavior, body patterning, growth and life history of Octopus briareus cultured in the laboratory. Am. Malacol. Bull. 7: 21-45.
Hanlon, R. T., M. J. Smale, and W. H. H. Sauer. 1994. An ethogram of body patterning behavior in the squid Loligo vulgaris reynaudii on spawning grounds in South Africa. Biol. Bull. 187: 363-372.
Hanlon, R. T., M. R. Maxwell, and N. Shashar. 1997. Behavioral dynamics that would lead to multiple paternity within egg capsules of the squid Loligo pealei. Biol. Bull. 193: 212-214.
Hanlon, R. T., M. R. Maxwell, N. Shashar, E. R. Loew, and K.-L. Boyle. 1999. An ethogram of body patterning behavior in the biomedically and commercially valuable squid Loligo pealei off Cape Cod, Massachusetts. Biol. Bull. 197: 49-62.
Jantzen, T. M., and J. N. Havenhand. 2003. Preliminary field observations of mating and spawning in the squid Sepioteuthis australis. Bull. Mar. Sci. 71: 1073-1080.
King, A. J., S. A. Adamo, and R. T. Hanlon. 1999. Contact with squid egg capsules increases agonistic behavior in male squid (Loligo pealei). Biol. Bull. 197: 256.
Larcombe, M. F., and B. C. Russell. 1971. Egg laying behavior of the broad squid, Sepioteuthis bilineata. N.Z.J, Mar. Freshwat. Res. 5: 3-11.
Lehner, P. N. 1979. Handbook of Ethological Methods. Garland STPM Press, New York.
Martin, P., and P. Bateson. 1993. Measuring Behavior: An Introductory Guide, 2nd ed. Cambridge University Press, Cambridge.
Mather, J. A., and D. L. Mather. 1994. Skin colours and patterns of juvenile Octopus vulgaris (Mollusca, Cephalopoda) in Bermuda. Vie Milieu 44: 267-272.
Messenger, J. B. 2001. Cephalopod chromatophores: neurobiology and natural history. Biol. Rev. 76: 473-528.
Moynihan, M. 1985. Communication and Noncommunication by Cephalopods. Indiana University Press, Bloomington.
Moynihan, M., and A. F. Rodanicne. 1982. The behavior and natural history of the Caribbean reef squid Sepioteuthis sepioidea. With a consideration of social, signal, and defensive patterns for difficult and dangerous environments. Adv. Ethol. 25: 1-151.
Packard, A. 1982. Morphogenesis of chromatophore patterns in cephalopods: are morphological and physiological ‘units’ the same? Malacologia 23: 193-201.
Packard, A., and F. G. Hochberg. 1977. Skin patterning in Octopus and other genera. Symp. Zool. Soc. Lond. 38: 191-231.
Packard, A., and G. D. Sanders. 1969. What the octopus shows to the world. Endeavour 28: 92-99.
Packard, A., and G. D. Sanders. 1971. Body patterns of Octopus vulgaris and maturation of the response to disturbance. Anim. Behav. 19: 780-790.
Peel, G. 2000. Comparative life history of tropical and temperate Sepioteuthis squids in Australian waters. Ph.D. thesis, James Cook University, Australia.
Peel, G. 2001. Flexible reproductive strategies in the tropical and temperate Sepioteuthis squids. Mar. Biol. 138: 93-101.
Porteiro, F. M., H. R. Martins, and R. T. Hanlon. 1990. Some observations on the behaviour of adult squids, Loligo forbesii, in captivity. J. Mar. Biol. Assoc. UK 70: 459-472.
Rice, W. R. 1989. Analyzing tables of statistical tests. Evolution 43: 223-225.
Roper, C. F. E., and F. G. Hochberg. 1988. Behavior and systematics of cephalopods from Lizard Island, Australia, based on color and body patterns. Malacologia 29: 153-193.
Segawa, S., T. Izuaka, T. Tamashiro, and T. Okutani. 1993. A note on mating and egg deposition by Sepioteuthis lessoniana in Ishigaki Island, Okinawa, Southwestern Japan. Venus Jap. J. Malacol. 52: 101-108.
Triantafillos, L., and M. Adams. 2001. Allozyme analysis reveals a complex population structure in the southern calamary, Sepioteuthis australis, from Australia and New Zealand. Mar. EcoL Prog. Ser. 212: 193-209.
Yang, W. T., R. F. Hixon, P. E. Turk, M. E. Krejei, W. H. Hulet, and R. T. Hanlon. 1986. Growth, behavior, and sexual maturation of the market squid, Loligo opalescens, cultured through the life cycle. Fish. Bull. 84: 771-798.
Whale Sharks – the gentle giants of NW Australia
by Evan and Kady John

The Whale shark (Rhincodon typus) is the world’s largest living fish. A huge and distinctively marked animal, it is found in tropical and warm temperate seas around the equator between 30o north and 35o south latitudes in both coastal and oceanic waters. Their range appears to coincide with areas where sea surface temperatures are between 21o and 25o C and cool, nutrient–rich upwellings mingle with warm, plankton-laden surface waters.
In Australia, whale sharks are found mainly off northern WA, Queensland and the Northern Territory, although some have been reported in NSW and Victoria. Their regular annual appearance along the Ningaloo Marine Park coastline, between Shark Bay and Exmouth in WA, has become one of the main tourist attractions of the region. The Ningaloo reef stretches 260 kilometres from North West Cape to Amherst Point, and is the only major reef in the world that is found on the west coast of a continent. Not only does the Leeuwin current flow across the top of Australia and down this coast from the Pacific Ocean, bringing warm water to the reef, but, in addition, the continental shelf is closer to the shore here than anywhere else along our coasts. Whale sharks congregate at spots on the Ningaloo reef’s seaward side, one of the very few places in the world (the Galapagos Islands is another where they appear regularly in any numbers between late March and July.

Whale shark (Rhincodon typus)
There is little doubt that they gather to feed, for their appearance is preceded by the spawning of over 200 species of coral 7 – 9 nights after the March and April full moons each year, setting off a chain of events that in turn increases local productivity within food chains; plankton and consequently small schooling fishes, sardines, anchovies and mackerel.

Whale shark feeding
Whale sharks are generally encountered singly, but aggregations of more than one hundred have been reported. They are frequently observed near the surface, where plankton and bait fish shoals are found, cruising along the surface with their large mouths agape, hence actively forcing thousands of litres of water an hour, containing small planktonic organisms such as krill and copepods into their mouths, straining them through a fine mesh of gill rakers which act like a large colander collecting the food at the back of their throat. The water then pours out through their ten gill slits. Although whale sharks have tiny teeth (3,000 – 5,000 in fact), arranged in more than 300 rows), they do not bite or chew their food, even though the plankton and crustacean diet is supplemented periodically with squid and small fish like anchovies and sardines.

Looking a whale shark in the eye!!
A fully grown whale shark can reach up to 18 metres in length. Most of those found at Ningaloo reef are between 4 and 12 metres. A 12 metre whale shark will weigh up to 11 tonnes, and will have a mouth as wide as one metre or more.
Very little is known about growth rates, size at sexual maturity, or how long they live, although it has been postulated that they may have a life span of more than 100 years, reaching maturity at 30 years. It is known that they are ovoviviparous, young hatching from egg cases inside the female and being born live, but the exact numbers of progeny is unclear, although in one instance, two biologists reportedly found 30 young whilst dissecting a dead animal taken from fisheries in Taiwan.
Unfortunately, some countries may still be killing these gentle giants for food and for other uses. Although the flesh is very soft and bland, they may still be the target for limited fisheries, possibly in India, Pakistan, Taiwan and China. Two Taiwanese fisheries are reportedly bringing in 60-100 whale sharks annually. Some are also accidentally caught in gill and purse seine net fisheries. There was a very limited traditional fishery for their liver oil in the Maldives - the liver oil has been used for waterproofing wooden fishing boats and other appliances. Some cultures also believe that there are cancer-healing properties in whale shark liver oil.

Kady with a Whale shark
Kady and I were very privileged to spend about two hours swimming with four 9 metre whale sharks in July last year on the Ningaloo reef. It is quite extraordinary slipping into the water, and then watching in awe as this seemingly immense animal rises slowly towards the swimmer. For Kady it was no doubt a little disconcerting in that the appearing mouth gape was almost as wide as she is tall!!
It was one of the most memorable events in our lives, and quite humbling in that these huge gentle creatures, that so easily could break bones with one quick swipe of their caudal fin seem quite content, even condescendingly interested, in having 1.8 metre creatures swimming with them for 30 minutes or so at a time.
My Continuing "Encounter" Experiences
by Steve Reynolds
In two previous articles I wrote about some of my personal experiences related to "Encounter 2002". The two articles were "After The Encounter" (7 parts - MLSSA Newsletters January to July 2003) and "The Encounter Continues" (MLSSA Journal No.13, December 2003). My experiences with Encounter related matters, however, do not stop there. They still continue to occur.
I would, however, first like to take a little step back. In January 2000 I revisited South Australia’s Kangaroo Island which had been named by Matthew Flinders in 1802. My wife and I boarded the Sealink ferry at Cape Jervis to reach the island. Flinders had also named the cape. He named it after Sir John Jervis on 23rd March 1802. The ferry crossed Backstairs Passage which Flinders had also named. He gave it that name because he considered it to be "a private entrance to the two gulfs". The ferry docked at Penneshaw where the Baudin Conservation Park is now located.

Southern Basketstar, under Penneshaw jetty
Photograph by David Muirhead
I have only ever managed to do just one dive at Penneshaw jetty. It was in April 1988 and that dive was cut short – see my report "Dive Cut Short At Penneshaw Jetty" in our April 2003 Newsletter (No.298).
One of our first activities on the island was to visit Frenchman’s Rock at Hog Bay near Penneshaw. This is the spot where one of Nicolas Baudin’s men inscribed a message onto a rock in 1803. The original rock is now located in the Gateway Information Centre at Penneshaw and a replica has been put in its place. The replica is now protected by a white dome-like structure. Several reasons for the naming of Hog Bay have been put forward. For example, it is said to have been named by sealers who found pigs roaming the area. Baudin had apparently left the pigs there as a food source for shipwrecked sailors. Then there is the story of a character called Governor Wallen who had pigs there in the 1820s. Another version tells of "the mysterious ways that pigs used to arrive at the spot (out of the sea?)".

Steve at Frenchman’s Rock at Hog Bay near Penneshaw
Photograph by Noeleen Reynolds
Penneshaw was named by a Mr HL Beddome in 1881/2. Beddome surveyed the town in 1881. It was then proclaimed in 1882. "Penneshaw" is a combination of the two names Pennefather and Shaw. Pennefather was Beddome’s Private Secretary and Shaw was a Miss FL Shaw who apparently was a frequent visitor at Government House. From Penneshaw we moved on to Christmas Cove, the site of my first dive on KI in 1988. It is a lovely little place. I enjoyed my dive there and the marine life was great. Matthew Flinders had apparently landed close to Christmas Cove in 1802. Surprisingly though, he didn’t give Christmas Cove its name. It is said to be named after an early settler. A plaque (on an obelisk?) commemorating Flinders’ landing was unveiled in 1936 during centenary celebrations. Granite boulders there were left by a glacier some 200m years ago.

Christmas Cove, near where Flinders landed
Photograph by Steve Reynolds
I don’t recall seeing the obelisk at Nepean Bay (near Christmas Cove) that commemorates Flinders’ landing on KI and naming it. Nor do I recall seeing a cairn at Kangaroo Head marking Flinders’ first landing. Our next main activity on the island was to climb Mount Thisby. Mount Thisby is a huge sand dune which Matthew Flinders had climbed in 1802. A Mr Thisby (or Tisby) is said to have had a camp on the hill. Flinders, though, had originally named the dune Prospect Hill and it regained that name on 4th April 2002, two hundred years later. Flinders had climbed the sand dune hill on 4th April 1802 and gave it its name due to the interesting view from its summit. Prospect Hill overlooks Pelican Lagoon which was named by Flinders. He described it as "a hidden lagoon of an uninhabited island".

View from the top of Mount Thisby (now Prospect Hill) overlooking Pelican Lagoon
Photograph by Noeleen Reynolds
Prospect Hill is situated on the narrowest part of KI and also overlooks the Southern Ocean. Flinders saw this view for himself and it convinced him that he was on an island. He never explored the southern coast of KI though, but Baudin’s expedition did in 1803. This is how D’Estrees Bay came to be named after Vice-Admiral Victor-Marie, duc de d’Estrees.
Prospect Hill is on the shore of Pennington Bay which had been named by Captain Bloomfield Douglas after a Joseph Pennington who was lost in scrub there in 1857.
We stayed at the Kingscote Caravan Park which overlooked Western Cove which Flinders had named due to its westerly location in Nepean Bay. Flinders had named the bay after Sir Evan Nepean on 21st March 1802.
During our next few days on the island we visited locations such as Bay of Shoals near Kingscote. Baudin had named the bay ‘Anse des Haute-Fonds’ (Shoal Cove).

Bay of Shoals
Photograph by Noeleen Reynolds
We visited Emu Bay and the jetty there. I don’t recall seeing any emus, Dromaius novaehollandiae there but we were fortunate enough to see an echidna, Tachyglossus aculeatus by the beach. It was named Emu Bay only in 1941 after originally being called Maxwell. Maxwell was planned as a main port and the jetty was built there around 1916. The water around Maxwell, however, was too shallow and, because it was subject to exposure to northerlies and easterlies, Kingscote became a port in its place. Emu Bay is close to Cape D’Estaing which was named by Louis de Freycinet after the French Admiral Charles Hector Theodat, Count D’Estaing.
We also visited the Seal Bay Conservation Park to see the Australian sea lions, Neophoca cinerea there. Seal Bay is on the west side of Cape Gantheaume and the Cape Gantheaume Conservation Park. The cape was named by Freycinet after Admiral JA Gantheaume (Vice-Admiral honore, comte Gantheaume?). We were also able to view the skeleton of a humpback whale, Megaptera novaeangliae in the sand dunes at Seal Bay. Seal Bay was first declared a sanctuary by the then Department of Fisheries and Game in 1954, thanks to lobbying by the Royal Society of SA’s Field Naturalists section. It was then rededicated as a fauna reserve in 1967. The waters in the bay became an aquatic reserve in 1971. Then in 1972 the area was rededicated as a conservation park.

Humpback whale skeleton in the sand dunes at Seal Bay
Photograph by Noeleen Reynolds
Then we went to see Vivonne Bay which Freycinet had named after Vice-Admiral Louis Victor de Rochechouart, duc de Mortemart et de Vivonne. The Vivonne Bay Conservation Park is located at Cape Kersaint which was named by Freycinet after the French naval family Armand, comte de Kersaint.
After seeing the Vivonne Bay jetty we moved on to the Kelly Hill Caves. These are in the Kelly Hill Conservation Park which is within the Cape Bouguer Wilderness Protection Area. The Protection Area surrounds Cape Bouguer itself which had been named by Freycinet after the French astronomer Pierre Bouguer.
We then visited the Flinders Chase National Park which had been named after Matthew Flinders. The National Park occupies the entire western end of the island. The park had first been declared a nature reserve in 1919, a sanctuary for wildlife. It came under the control of the National Parks & Wildlife Service in 1972.
We were able to see the sights at Cape du Couedic, including the granite boulders called Remarkable Rocks, the lighthouse, Admirals Arch and the nearby Casuarina Islets. Admirals Arch is a spectacular natural arch below the Cape du Couedic lighthouse. We were able to watch New Zealand fur seals, Arctocephalus forsteri under the arch. The Cape du Couedic lighthouse was opened in either 1906 or 1909.

Admirals Arch, Cape du Couedic
Photograph by Jo Lush
Louis de Freycinet named the rocky Cape du Couedic after the French Navy Captain Chevalier du Couedic. In 1935 the steamship Portland Maru hit a submerged reef near Cape du Couedic and began sinking. It had to be beached between Cape Borda and Cape Torrens. Cape Borda was named by Freycinet after John-Charles de Borda, a mathematician and naval officer.
The Casuarina Islets were named after Freycinet’s ship, the schooner Casuarina. The islets are now a bird sanctuary.

View of the Casuarina Islets from Cape du Couedic
Photograph by Steve Reynolds
Before leaving the island we managed to visit American Beach which has since been renamed Baudin Beach after Nicolas Baudin. The renaming took place on 4th April 2002 at the unveiling of a sculpture of Mary Beckworth who had been mistress to Baudin onboard the Geographe.
We managed to view the old jetty at Reeves Point. This was the first jetty built on KI. Reeves Point was the site of the first formal settlement in SA. The South Australian Company settled it in July 1836. A jetty was built on a promontory of land dividing the Bay of Shoals from Nepean Bay proper. The jetty was finished late in August 1838. Funny thing that because Reeves Point is named after a settler called Augustus Reeves.

The old jetty at Reeves Point, near Kingscote
Photograph by Steve Reynolds
The Reeves Point site was first called Kingscote after Henry Kingscote of the South Australian Company. In 1883 a new town called Queenscliffe was surveyed. In 1940 Queenscliffe became the new Kingscote and the old Kingscote became Reeves Point.
I managed to dive under the Kingscote jetty in the waters of Nepean Bay on 8th January 2000, 197 years after the Geographe and the Casuarina had been anchored in the area. I also visited Jenny Clapson’s gallery nearby. It is now called the Kangaroo Island Marine Centre and has aquarium displays featuring local marine life. Jenny now sponsors our Society’s Newsletter.

Kingscote jetty and Jenny Clapson’s "Kangaroo Island Marine Centre"
Photograph by Steve Reynolds
Our last visit to Kangaroo Island was, of course, before the "Encounter 2002" celebrations. Since those celebrations I have been able to revisit Flinders memorials at places such as Port Wakefield. There is one by the swimming lake close to the creek in the town. Then there is another one close by, on the road through the South Hummocks from Port Wakefield to Kulpara. The spot overlooks Gulf St Vincent, named after Lord St Vincent by Flinders. It also overlooks Yorke Peninsula, named by Flinders after the Right Honourable Charles Philip Yorke of the Admiralty on 30th March 1802.
Flinders had also named the Hummocks in 1802. He is said to have bestowed the name "Hummock Hill" on the " ‘hummocky mountain’ at the head of the gulf (St Vincent)". However, he also named a "Hummock Hill" at Whyalla, being "the furthest hummock seen from the anchorage".

Flinders Memorial near the South Hummocks
Photograph by Steve Reynolds
In January 2004 we visited the Limestone Coast in the southeast of SA where we were able to experience even more Encounter related matters. After leaving Adelaide, we drove to Murray Bridge and across the Murray River. The river was missed by both Flinders and Baudin in 1802, largely due to their chance encounter nearby. After Tailem Bend we turned towards the coast to head for Meningie on the Princes Highway. Meningie had been surveyed by a W.Farquhar in 1866. A W.Farquhar was also my dive instructor and club President 122 years later.
The Princes Highway follows the Coorong coast down to Kingston. Some maps show the Coorong to be on the shores of Encounter Bay, which Flinders had named after his friendly encounter with Baudin. Other maps, however, show Encounter Bay on the west side of the Coorong and Lacepede Bay on the east side of the Coorong.
We stayed at the Cape Jaffa Caravan Park for our visit to the southeast of SA. Cape Jaffa is situated on the shores of Lacepede Bay, between Kingston SE and Robe. Freycinet named the bay after Count Bernard Germaine Etienne de la Ville Lacepede who was a French naturalist and ichthyologist.
Matthew Flinders is said to have incorrectly* named the spot Cape Jaffa, the name given by Baudin for what is now Cape Martin at Beachport. Louis de Freycinet’s charts named the cape at Cape Jaffa "C.Bernouilli", possibly after Daniel Bernouilli who was a Swiss mathematician. Bernouilli was born in 1700 and became a Professor in maths, anatomy, physics and botany. He died in 1782. Flinders applied the name Bernouilli to a small cape on the Coorong coast*. The name later disappeared from maps.
* According to Geoffrey H. Manning in his book "The Romance of Place Names of South Australia", Flinders had noted in his journal "The succeeding part of the (SE) coast having been first discovered by the French navigator (Baudin), I shall make use of the names . . . . I must be excused should any errors be committed in the nomenclature."
De Freycinet’s "Cape Bernouilli" was used for today’s Cape Jaffa until the mid-1850s. The cape is said to be named after Israel’s Jaffa (or Yafo or Joppa). It is the "Joppa" referred to in the Bible’s Old Testament. It is a port on the Mediterranean coast close to Tel Aviv. Jaffa is a generic word for "beauty" in several ancient languages. It can mean ‘fine’ or ‘beautiful’. Napoleon Bonaparte apparently took Joppa (or Jaffa) in 1799, the year that he became France’s First Consul.
After checking in at the Cape Jaffa Caravan Park, we had to head straight to Beachport which is a former whaling port. Beachport was named after Sir Hicks-Beach, Secretary of State for the Colonies. It is on the shores of Rivoli Bay. Freycinet named the bay after Andrea Massena, the Duke of Rivoli, said to be Napoleon’s greatest General. Rivoli was apparently a Napoleonic battlefield. Whilst at Beachport I was able to do a quick dive in the waters of Rivoli Bay, under the 772m-long jetty, the second longest jetty in SA.

Beachport’s temporary boat ramp with jetty in background
Photograph by Noeleen Reynolds
The original "Cap de Jaffa" was southwest of the jetty. South from Beachport at the southern end of Rivoli Bay is Cape Buffon where Southend is located. Cape Buffon is named after the French naturalist George Louis Leclerc, Comte de Buffon. There has been much confusion over Cape Buffon though, with Freycinet wanting to call it Cape Lannes, a name now applied to the site of the Robe lighthouse.
Cape Buffon is also the northern tip of the Canunda National Park which stretches down to the southern tip of Lake Bonney and the Cape Banks lighthouse which was de-manned in 1928.
As we headed back to the Cape Jaffa Caravan Park, we drove through the Mount Benson Wine Region. Cape Jaffa Wines are located in the area. The region is just north of Guichen Bay which had been named by Freycinet. It was named in honour of French Admiral de Guichen who had apparently fought several battles against the British.
The Baudin Rocks Conservation Park is offshore from Guichen Bay. The rocks were named by Flinders in honour of Nicolas Baudin, but back to the Mount Benson Wine Region. It has a mild, maritime climate and southern ocean breezes. It has terra-rossa soil over limestone and produces rich, full-bodied fruit with distinct character. The Guichen Bay Vineyard is in the Mount Benson Region. Baudin Rock Wines are produced at the Baudin Rock Vineyard which nestles high atop the hills of the region.
Our second day in the South-East was mostly spent travelling to (and from) Naracoorte where we explored the magnificent caves there. On our way back to the caravan park, however, we drove through Kingston where we visited the 17m-high Larry (the big) Lobster (or giant cray).

Steve under Larry the Lobster (Jasus edwardsii?)
Photograph by Noeleen Reynolds
We then drove along Marine Parade on the shore of Lacepede Bay. We saw the old Cape Jaffa lighthouse there. The lighthouse had originally been built on the Margaret Brock Reef between 1868 and 1872. The reef is named after the barque Margaret Brock which was wrecked there in 1852. The Margaret Brock is a protected shipwreck under the Commonwealth Historic Shipwrecks Act 1976.
We returned to the old Cape Jaffa lighthouse the next day for a guided tour. The lighthouse had been rebuilt at Kingston in 1975. The former platform for the lighthouse at the Margaret Brock Reef is due to be dismantled at the time of writing due to safety issues.
We spent the rest of the day exploring Kingston and Cape Jaffa, including their jetties. Kingston is said to be a conglomerate of some four towns. At Cape Jaffa we walked around the cape itself where we could look south towards the Bernouilli Conservation Park. There are several offshore cages for breeding Atlantic Salmon at Cape Jaffa.

Kingston jetty
Photograph by Noeleen Reynolds
On the fourth day we visited the town of Robe at the southern end of Guichen Bay. Robe is named after Lieutenant-Colonel Frederick Holt Robe. Robe was SA’s 3rd Governor and he chose the area as a port while on a survey voyage in 1845.
15km southeast of Robe is the Waterhouse Range Vineyard which produces the Governor Robe Selection label wines. The label had been launched by SA Governor Marjorie Jackson-Nelson during the Encounter 2002 celebrations in Robe. The Governor Robe Selection name had been chosen to honour Governor Robe. The shore of Guichen Bay is mostly taken up by the 17km-long Long Beach. At the northern end of the beach is the Guichen Bay Conservation Park. Baudin Rocks are due west of the park. Although we spent much of the day gazing out at the rocks, we still managed to visit the Robe Visitor Information Centre where we were able to read a little about Flinders and Baudin.
We then visited the "Royal Circus" in the middle of Robe. There was a flagstaff there at the spot from which Governor Robe and Thomas Burr, the surveyor took the first theodolite bearings for the town in 1846. There is a cannon said to be from the barque Koenig Wilhelm II (some say Koning) which was wrecked in Guichen Bay in 1857. Then there is the Matthew Flinders Memorial Seat which commemorates Flinders’ survey of the coast in 1802.

The Matthew Flinders Memorial Seat at Robe
Photograph by Steve Reynolds
From the middle of the town we headed past the beautiful Lake Butler Boat Haven towards the rocky coast. We went to the site of the Robe lighthouse at Cape Lannes, named by Freycinet after Jean Lannes, Duc de Montebello, Marshall of the French army. Freycinet’s Cape Lannes, however, was the spot now known as Cape Buffon. The lighthouse was built in 1972 to replace the Cape Jaffa lighthouse. It is an automatic, unmanned lighthouse. We then stood on Factory Beach to view the waves of the sea crashing through "Doorway Rock".

Doorway Rock as seen from Factory Beach at Robe
Photograph by Steve Reynolds
Next we visited the Old Gaol ruins close to Cape Dombey, named after Joseph Dombey, a French botanist and physician. The Old Gaol was used to stage an outdoor opera on 13th April 2002. I noticed that some ironwork from the wreck of the steamer Admella had been used to reinforce some of the gaol’s walls. The Admella is a protected shipwreck under the Commonwealth Historic Shipwrecks Act 1976. The name of the Admella comes from the combination of its three main destinations – ADelaide, MELbourne and LAunceston. The Admella was wrecked at Carpenter Rocks in 1859 with the loss of 86 lives. Baudin had named the rocks "Les Charpentiers" (The Carpenters) because they were indented like the teeth of a saw.
The famous Robe obelisk built between 1852-5 was nearby. It’s an impressive 12m high. I later read that it had been recently repainted in its red and white colours for the "Encounter" celebrations in 2002.

The obelisk at Cape Dombey, Robe
Photograph by Noeleen Reynolds
From the site of the obelisk, we took a walk along a part of the Cape Dombey Walking Trail back towards the local jetty. The walking trail is one of several Encounter trails. It was opened on 13th April 2002. The trail and interpretive signage was funded through Coastcare, Encounter 2002 and the District Council of Robe.

View of Robe jetty & Guichen Bay from the Cape Dombey Walking Trail
Photograph by Noeleen Reynolds
I wanted to do a dive in the waters of Guichen Bay under the Robe jetty but it was very dirty water and access was virtually non-existent. I probably could have jumped in but I couldn’t see a way out again other than to swim back to where waves were crashing onto the rocky shore.

Close-up of Robe jetty, Guichen Bay (built 1866?)
Photograph by Steve Reynolds
We visited the boat ramp at the boat haven but missed seeing the Fisherman’s Memorial there. We then returned to Cape Dombey to photograph the "Encounter Signal". It is a modern sculpture built for the Encounter celebrations. It pays tribute to Robe’s maritime tradition and Flinders and Baudin. Our Governor, Marjorie Jackson-Nelson had opened the sculpture on 13th April 2002. The opening marked the closing of Encounter 2002 celebrations in SA.

The Encounter Signal at Cape Dombey, Robe
Photograph by Steve Reynolds
When we returned to Cape Jaffa I went for a walk along the jetty there. The jetty had been built in 1868. I wanted to see if it was worth going for a dive (just because I wanted to do a dive in the waters of Lacepede Bay). The wind that had been blowing for the past three or four days had died down considerably. I found that the water was quite dirty though and access was once again difficult.
Only one thing remained for me to do then whilst I was at Cape Jaffa. That was for me to take a photograph of the Seafarers’ Memorial close to the jetty. The stone cairn memorial was dedicated during Encounter 2002 celebrations. It honours the fishermen, seafarers and lighthouse keepers who lost their lives near Cape Jaffa. There are three separate plaques with a total of 34 names. There is a book by Millicent historian John Nicholson about the stories behind the memorial. It is "Cape Jaffa – Its Memorial To Seafarers, Fishermen and Lightkeepers".

The Cape Jaffa Memorial To Seafarers, Fishermen and Lightkeepers
Photograph by Steve Reynolds
All too soon, it was time to drive back home to Adelaide. It was a pity that we couldn’t stay any longer because the Cape Jaffa Seafood & Wine Festival 2004 was being held two days later. The festival gives visitors the chance to taste and enjoy local food, wine and jazz music. The festival apparently won an award in 2001.
They say that there are wrecks and limestone reefs for divers to explore at Cape Jaffa. I can now only dream about diving at places such as Baudin Rocks and the Margaret Brock reef. They say that the underwater scenery at the reef is spectacular.
We have been able to revisit the permanent Flinders and Baudin display at the SA Maritime Museum at Port Adelaide. This is the display described in my article "The Encounter Continues" in our 2003 Journal. We were also able to revisit the Matthew Flinders statue which stands in North Terrace, Adelaide. This is the statue pictured on page 10 of our March 2003 Newsletter (No.297). It was sculpted by a Frederick Brook Hitch and was unveiled in 1934.
We have also revisited the Encounter display at the SA Whale Centre at Victor Harbor. Late in 2003 I visited the Encounter monument at Victor Harbor (Sorry, no photos were taken this time). This is the ‘three poles’ that I described in our 2003 Journal. It had been unveiled by former French Prime Minister, Michel Rocard late in March that year.
Every time I do a dive in either Gulf St Vincent, Spencer Gulf or Encounter Bay I am visiting the waters explored by Messrs. Flinders, Baudin and Freycinet and named by Flinders. The same applies whenever I dive from either the Fleurieu Peninsula or Yorke Peninsula since these were named by Freycinet and Flinders.
Freycinet named the Fleurieu Peninsula after Charles Pierre Claret, Comte de Fleurieu who had been French Minister for the navy under Napoleon. He financed Baudin’s expedition on the Geographe. He was a member of the Council which prepared instructions for the voyage. The name of the peninsula was bestowed in 1911 after a grandnephew of Claret had visited SA. Freycinet had wanted to name Cape Jervis ‘C.Fleurieu’.
Whenever I visit Mount Lofty I am in the area named by Flinders on 23rd March 1802 when he saw it from Kangaroo Island. I have, however, read that Capt. Collet Barker was credited with naming Mount Lofty when he visited the area in 1831.
I returned to Mount Lofty on 26th January 2004 and revisited the summit there to inspect Flinders’ Column (Sorry, no photos were taken this time either). The column was built as a trignometrical station in 1885 but in 1902 it was named in honour of Flinders to mark the centenary of his sighting of Mount Lofty from Kangaroo Island. It seemed appropriate that I was there on Australia Day since Flinders had fought for the use of the name "Australia" until his death in 1814. Governor Macquarie started to use the name after reading Flinders’ book in 1817. Phillip Parker King, Flinders’ successor in surveying the Australian coast was able to use the name on his charts by 1827.
I am unsure whether I visited the Flinders Lookout at Whyalla when I visited the area in 1988. I think that I did. It became the Flinders – Freycinet Lookout in September 2001. Whilst on the subject of Whyalla, while I was there in 1988 I dived two or three times at Point Lowly. This descriptive name was given by Flinders on 9th March 1802.
Further "Encounter Events"
"Encounter" events that have occurred since my article "The Encounter Continues" (MLSSA Journal No.13, December 2003) include the launch of the English translation of Francois Peron’s "Voyage of Discovery to the Southern Lands (Vol. II)". It was published by the Friends of the State Library late in 2003. There were two editions published – a deluxe edition and a standard one, the latter priced at $115.
In August 2003 a play called "The Stowaway and the Captain’s Cat" was showing at the Dunstan Playhouse. The play, written by Anne Brookman, was all about Flinders, his cat Trim and a stowaway cat.
Twentyeight scientists took part in an expedition to the Nuyts Archipelago in 2002. They discovered eight new species of jellyfish. The results of the expedition were released in the "South Australian Geographical Journal", the Journal of the Royal Society of South Australia. These details were reported in our February 2004 Newsletter (No.307). Four of the new jellyfish species were named after the SA Research and Development Institute (SARDI), two SARDI personnel and the SARDI research vessel Ngerin. Hexaphilia scoresbyi was named after Dr Scoresby Shepherd, the SARDI honorary research fellow and MLSSA Patron. Amphinema cheshirei was named after SARDI Aquatic Sciences Chief Scientist, Professor Anthony Cheshire. Zanclea sardii was named after SARDI itself and Zanclea ngeriana (I hope that’s right) was named after the SARDI research vessel RV Ngerin.
Flinders named the Nuyts Archipelago after Pieter Nuyts, supercargo on theDutch ship called the Gulden Zeepaard in 1627. Super cargo is ‘an officer of a merchant ship superintending (sales of) cargo’. Nuyts was an official for the Dutch East India Company (Council of the Indies). He sailed 1000 miles (1500kms) along the southern coast of Australia in the Gulden Zeepard (Golden Seahorse) in 1627.
I have seen "Zeepaard" spelled a number of ways including Zeepard, Seepaart, Seepaard and Leepard. "Nuyts" is also sometimes spelled ‘Nuijts’. Then there is the suggestion that the name should actually be either Nuyt or Nuijt and that the addition of an ‘s’ without an apostrophe is simply a case of bad grammar. As discussed in my article "Nicolas Baudin’s Scientific Expedition To The Terres Australes" (MLSSA Journal No.12, December 2001) "When the captain of the Gulden Zeepard . . . decided against continuing further and turned his ship back around he named the area Pieter Nuijts Land. Although this name has been anglicized to become Peter Nuyts Land, the positioning of an apostrophe is questionable." Perhaps the name should be Nuijt’s or Nuyt’s Land.
PICTURE CREDITS
Steve and Noeleen Reynolds
David Muirhead - Southern Basketstar
Jo Lush - Admirals Arch
REFERENCES:
"Nicolas Baudin’s Scientific Expedition To The Terres Australes" by Steve Reynolds, MLSSA Journal No.12, December 2001.
"After The Encounter" by Steve Reynolds, (7 parts) MLSSA Newsletters Jan to July 2003.
"The Encounter Continues" by Steve Reynolds, MLSSA Journal No.13, December 2003.
"Cape Jaffa – Its Memorial To Seafarers, Fishermen and Lightkeepers" by John Nicholson (2002).
"The Romance of Place Names of South Australia" by Geoffrey H. Manning (1986).
"South Australian Geographical Journal", Special issue for Encounter 2002, Vol.99, 2000, Royal Geographical Society of South Australia.
by
Reefwatch personnelThe 2004 Reef Watch "Marathon" dive took place at Noarlunga Reef on Sunday 14th March and involved 50 divers and 20 snorkellers. In total there were 41 fish surveys and 18 benthic surveys (LITs were done for the first time on a marathon dive). There was significant media coverage of the event.
 Half of the 50 divers had been previously trained in the survey methods, while the other half received training on the day, as part of the PADI Reef Watch Survey Diver Specialty Course. For Tim Woonton and Maggie McGilchrist, it was their fourth and final dive of this course which now has 28 graduates and 88 other divers at various stages.
Half of the 50 divers had been previously trained in the survey methods, while the other half received training on the day, as part of the PADI Reef Watch Survey Diver Specialty Course. For Tim Woonton and Maggie McGilchrist, it was their fourth and final dive of this course which now has 28 graduates and 88 other divers at various stages.
 The event could not have taken place without the involvement of the dive instructors and divemasters, namely Dave Albano, Andy Davoren, Christopher Deane, Kevin Smith and Nick Turich, and of course Mary-Anne Stacey who ran her boat from dawn to dusk. The assistance of Daryl Metters, Patsy Mendham, Judy Kirkman, various members of the steering committe and many other volunteers was also vital.
The event could not have taken place without the involvement of the dive instructors and divemasters, namely Dave Albano, Andy Davoren, Christopher Deane, Kevin Smith and Nick Turich, and of course Mary-Anne Stacey who ran her boat from dawn to dusk. The assistance of Daryl Metters, Patsy Mendham, Judy Kirkman, various members of the steering committe and many other volunteers was also vital.
The kits used for the monitoring were the result of a grant of $1800 from the PADI Project AWARE Foundation. The event was also supported by Port Noarlunga Dive and Snorkel Centre, Underwater Sports, Southern Diving, Dolphin Dive and Divers Delight, who provided various incentives and discounts to participants. Dolphin Dive have agreed to give ongoing such support to the program, and discussions are continuing with the metropolitan dive shops.
The data gathered was entered on the day or shortly after, and is currently being processed, along with data from previous marathon dives in 2000, 2001 and 2002. The results are being compared with those of scientific studies performed for the EPA by Adelaide and Flinders Uni in 1996 and 1999 (see the Reef Watch website www.reefwatch.asn.au, "Reef Health Monitoring -> Reports" section for these reports).
 A more complete analysis of the data from Marathon dives and the conclusions reached is being compiled into a report that will be freely available. Articles are also being prepared for Southern Fisheries, EcoVoice and numerous other publications. For now, we will present a subset of the results.
A more complete analysis of the data from Marathon dives and the conclusions reached is being compiled into a report that will be freely available. Articles are also being prepared for Southern Fisheries, EcoVoice and numerous other publications. For now, we will present a subset of the results.
http://www.underwatersports.com.au/t_blank
The following map is to assist interpretation of the results:
Fish
One simple yet interesting finding from the fish data collected during the 2001, 2002 and 2004 Marathon dives is the total number of fish species ("species richness") observed in different parts of the reef. This is shown in the graph below:
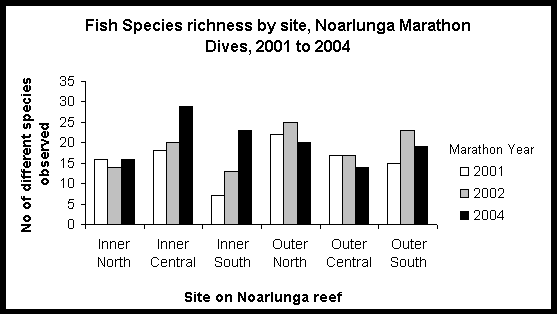
If you look at the numbers of fish species in 2001 (white bars) there appear to be more fish species present at the northern outside site than at other sites. The pattern is quite similar for the 2002 (grey bars) marathon dive. This consistency in the data, for most of the sites across several marathon dives, shows that we can be fairly confident in the quality of the data.
The observations for 2004 showed some slightly different trends. Firstly there were 29 species observed at the central inside site compared to 20 in 2002 (although the fish count methodology had changed during 2003, with a second or return pass for recording fish under the canopy or ledges, the data from this second pass was not included for this comparison).
 One might argue that this difference of nearly 45% is due to volunteers improving their fish identifying skills over the years! This may be true to a certain extent but there were also fewer species recorded in 2004 at each of the outer reef sites than in 2002.
One might argue that this difference of nearly 45% is due to volunteers improving their fish identifying skills over the years! This may be true to a certain extent but there were also fewer species recorded in 2004 at each of the outer reef sites than in 2002.
Not all of the sites were surveyed in 2000 and for clarity those data were not presented in the graph above, however, for each of the 3 outer sites that were surveyed in that year there were far fewer fish species recorded than in every subsequent marathon dive.
While there are a few different factors which affect the way this information can be interpreted there does appear to be a trend of improving fish species diversity over the five years that volunteers have been collecting information during Marathon dives.
Benthic Habitats
The report currently being prepared about the Reef Watch marathon data will show the current status of the Noarlunga benthic reef habitat, and will explore any links between this and the results of the most recent fish survey. It will also describe in general terms how different parts of Noarlunga Reef have changed over the last decade. Data from two sources will be used:
Here is some preliminary information about the change to one section of the reef, the northern outside reef (NNO), over the last eight years. The graph below indicates how the bottom cover is divided up between the various lifeforms identified during the surveys (totalling 100% for each year surveyed). For example, the top part of each graph is the large canopy algae (in blue for those of you that are reading the colour PDF version). You can see that it accounted for about 25% of the cover in 1996, about 65% in 1999, and then about 45% in 2001 and 2004. Also, there was almost no bare substrate (rock) in 1996 or 1999, but it increased to about 10% in 2001 and 20% in 2004.
Changes in the composition of Noarlunga North Outside NNO over the last decade. Data for 1996 and 1999 were obtained from the Reef Health Surveys commissioned by the EPA while more recent data (2001, 2004) were collected by Reef Watch volunteers.
 Note: given that the LIT method records the cover of organisms from above, it is biased towards the canopy lifeforms. As a result, if there are a lot of large algae, smaller lifeforms (e.g. LOBE algae, or STAR or SNAIL invertebrates) may not be recorded because they weren’t visible below the canopy. It does not mean that they weren’t there! If the quadrat method were used instead, those organisms would have been recorded. The LIT method does however have a number of other advantages that make it a more reliable method overall.
Note: given that the LIT method records the cover of organisms from above, it is biased towards the canopy lifeforms. As a result, if there are a lot of large algae, smaller lifeforms (e.g. LOBE algae, or STAR or SNAIL invertebrates) may not be recorded because they weren’t visible below the canopy. It does not mean that they weren’t there! If the quadrat method were used instead, those organisms would have been recorded. The LIT method does however have a number of other advantages that make it a more reliable method overall.
How Noarlunga Reef Has Changed
To understand the changes to a particular reef or section of reef, it is important to know what was happening in the wider area. According to available data, macroalgal cover on most of the Gulf St Vincent reefs was less during the mid 1990s than at the end of the decade, hence the change from 25% large canopy algae to 65% in the 1990s on the outer north section of Noarlunga Reef. This is believed to be the result of variations in climate (more information can be found in the 2004 PhD thesis by Reef Watch Chairman David Turner).
 Reef Watch divers have detected a reduction back to about 45% during 2001 and 2004 on the outer north section of Noarlunga Reef. This is believed to be the result of changed conditions on the reef arising from increased sedimentary impacts (see David Turner’s thesis).
Reef Watch divers have detected a reduction back to about 45% during 2001 and 2004 on the outer north section of Noarlunga Reef. This is believed to be the result of changed conditions on the reef arising from increased sedimentary impacts (see David Turner’s thesis).
Another scientific reef health survey is being planned for the end of this year with funding from a number of state and federal agencies. This will provide an opportunity to validate the findings of Reef Watch divers.
Importance of these findings
The large canopy algae described above are an important part of the reef ecology, providing lasting homes for a number of other species. The replacement of these large brown algae with bare space can be taken as a sign of degradation, and reefs under such stress may be in danger of losing some of their diversity.





Marathon Dive Sponsors
Extensions To Pt Vincent Marine Studies Centre
by Scoresby Shepherd
On 22 July last I attended the formal opening of extensions to the Marine Studies Centre at Pt Vincent Primary School. Although it has only a few students, the Centre is unique as an important teaching facility in marine studies for the whole of Yorke peninsula. The extensions now boast an impressive display of a large number of temperate and some tropical molluscs, which the children are putting in taxonomic order. In addition there is a growing library, and several first class marine aquaria displaying common species found in coastal waters.
The official opening, attended by local and State representatives, was actually orchestrated by the children (under the watchful eye of the Head), and they performed splendidly.
Before the opening I took the opportunity to dive on the School's adopted reef, a few km north of the town. It is a low algal-covered reef with a few caves and crevices, and gives plenty of scope for practical training in monitoring.
With both a practical and curriculum-focused teaching agenda in marine studies, this school is undoubtedly a jewel in the crown of school education. Congratulations to all at the school.
(Editors note: students of the Port Vincent Primary School have been monitoring "Golf Course" reef for the last 6 years using the Reef Watch fish count method).
(also appeared in Reef Watcher).