Marine Life Society of South Australia Inc.
2005
Journal
NUMBER 15
CONTENTS
On Some
South Australian Caulerpa Species
Preliminary
Field Observations of Mating and Spawning in the Squid
Sepioteuthis australis
More Nudibranch Discoveries
Recent Requests to Our Society for Information
Further Recent Requests to Our Society for
Information
EDITORIAL
- Philip Hall
Welcome
to the 2005 edition of the MLSSA Journal. As usual this replaces the December
monthly Newsletter.
This
edition of the Journal starts with an article containing a somewhat
controversial view of the benefits of Caulerpa taxifolia by MLSSA member
Brian Brock.
The
third and final part of the squid series of articles we have printed over the
past two years by Troy Jantzen and Jon Havenhand follows.
Steve
Reynolds contributes a beautifully illustrated article on local nudibranchs and
follows with an article on information requests received by the Society.
I
have added a second part to the information requests with some that I have answered.
DISCLAIMER
The
opinions expressed by authors of material published in this Journal are not
necessarily those of the Society.
EDITING: Philip
Hall
PRINTING: Phill
McPeake
CONTRIBUTORS: Brian
Brock
Troy
Jantzen &
Jon
Havenhand
Steve
Reynolds
Philip
Hall
PHOTOGRAPHY: Dennis
Hutson
Yoshi
Hirano
Philip
Hall
On Some South Australian Caulerpa Species
by B. J.
Brock BSc, Dip Sec Ed, MSc,
FRSSA, MAI Biol
The genus
Caulerpa includes a large number of bright green stoloniferous algae
bearing erect axes from which small side-branches called ramuli arise. The size, shape and arrangement of the ramuli on the axes, aids in
the identification of the species. Fine root-like structures called
rhizoids attach the stolons to the substrate. In some species, the primary axis
branches and the branches also have ramuli on them. Refer to Womersley’s habit
photographs and drawings of ramuli, and descriptions etc. if you wish to try
your hand at keying out species (Womersley H. B. S.; 1984, pp. 253-274)
Drift
specimens of Caulerpa may be found on many of our beaches after storms.
Shepherd and Womersley (1970) record summer shedding of fronds of two Caulerpa
species, leaving persistent stolons. Some species may be found attached in
Intertidal pools or in the upper sub-littoral. A few species occur over a
considerable range of depths. Caulerpa cactoides is a fairly robust
species with clavate ramuli. Fragments of it are quite often washed up after
storms, and I have seen it growing in several places in the upper sub-littoral
zone (Port Douglas near Coffin Bay, Marion Bay, Groper Bay near Pondalowie,
Marino Rocks, Edithburgh) and thought of it as a fairly shallow-water species.
However, systematic collecting on the Nuyts Archipelago found it in the 32-38
metre zone and also at 2 metres in a sheltered site (See: Shepherd S. A. and
Womersley H. B. S., 1976, p.188). Lucas (1936) p.48 records it “from low water
mark to a depth of several fathoms”.
The Port
Douglas form of Caulerpa cactoides differs from the Marion Bay form. One
illustration accompanying this article is a photo-copy of a herbarium sheet
prepared from a specimen collected from the blue-line off the Investigator Base
Campsite near Black Springs (ANZSES Expedition) by Lynn Brake on 30/12/83.

Picture 1
Port Douglas form
of C. cactoides
Compare it
with the Marion Bay form.

Picture 2
Robust Marion Bay
form of C. cactoides
Another species with a wide range of
depth distribution is Caulerpa scalpelliformis. The scuba divers
collected it on the four different transects in the St Francis group of islands
at depths from 2-35 metres (Shepherd and Womersley 1976). I found it upper
sub-littoral at the base of calcareous and harder metamorphic rocks near the
Black Springs base camp. In this situation, it had to tolerate underwater
sandstorms when there was much wave action. It could be confused with C.
taxifolia but the ramuli in C. taxifolia are in opposite pairs (see
Pic 3) while those of C. scalpelliformis alternate along the axis. The
shapes of the ramuli also differ slightly in the two species. Refer to the West
Island, Pearson Island and St Francis papers for accounts of algal ecology.

Picture 3
C.
taxifolia from Angas Inlet.
Ramuli in
opposite pairs on erect fronds.
Caulerpa trifaria
is a fairly delicate Caulerpa species that used to be popular in
marine aquaria. Three rows of fine ramuli run up the erect axes. The photo (Pic
4) shows the habit of a specimen collected at West Beach in 1975 by my
daughter. Fresh drift specimens of marine algae can be rinsed in fresh water
and floated out on cartridge paper. Dry between newspapers under weight. Muslin
over the specimen will prevent it from sticking to the newspaper, but papers
must be changed frequently to avoid mildew. Some dry reds are beautiful (Pic 5)
and keep for years, especially if stored in a cool, dry, dark place.

Picture 4
C. trifaria has
three rows of fine ramuli.

Picture 5
A herbarium sheet
of the lovely red alga Kallymenia tasmanica
Caulerpa
taxifolia (Aquarium Weed), is the species that initiated the battle
to contain it in West Lakes. That battle has been lost. Like Barley Grass and
other weeds on land, I think we will have to learn to live with it (and be
thankful that it is a photosynthetic organism that fixes a lot of solar energy
that passes on down whatever food chains). Some time ago, I saw what I thought
might be Caulerpa taxifolia growing near an old dead mangrove tree lying
near the end of the Fishermen’s jetty in the Angas Inlet. On 8/8/05, I decided
to check it out more closely, from the shore. It had gone, but just around the
corner, in a small inlet, was a knee-deep patch of suspicious-looking weed. I
know this inlet well because I used to find the upside-down jellyfish
Cassiopea ndrosia (Plates 15,3 and 15,4 of
Shepherd and Thomas 1982) here in thousands before the blooms of red tide
organisms began. The floor of the little inlet, used to be bare of weeds. A
sample of the weed-patch proved it to be a mix of Caulerpa taxifolia and
a recently introduced Western Australian species called Caulerpa racemosa (see
p. 270 and fig 91B of Womersley H. B. S. 1984; also the photo Pic 6
P 10 accompanying this article). I believe a similar mixed crop of weeds
is found along the Port River side of Torrens Island. This is not surprising
considering the way water circulates around Garden Island on the ebb and flow
of the tides. (Cold water from North Arm is used for cooling purposes by the
Torrens Island Power Station.) North Arm and Torrens Island are connected by a
causeway that separates the cold North Arm waters from the warm effluent
discharged into Angas Inlet. In times of peak demand, remote sensing of surface
water temperature (Thomas et al. 1986 fig.4) shows that some of the warm
effluent completely circles Garden Island. The warm effluent affects the
distribution and abundance (Thomas I. M. et al., 1986) and seasons of
settlement (Brock B. J. Unpublished M.Sc. thesis; and 1985) of some marine
invertebrate species.
I collected Caulerpa racemosa and an Ulva
species from the upper sublittoral in a mooring basin at the junction of
the Port River and North Arm (near the open air produce market) on 24/3/04. I
was able to key out the Caulerpa as C. racemosa using Womersley
(1984). Bob Baldock of the State Herbarium, verified
my identification on 26/3/04 and told me he had seen it on a collecting panel
about 1½ years previously. I mounted my specimen for the Maritime Museum’s
Dolphin display. Steve Reynolds of the Marine Life Society of South Australia
picked the herbarium sheets and drawings up on 2/4/04. Bob and I went to Angas Inlet, and the North Arm/Port River site on 5/4/04 for more
specimens of C. racemosa for the State Herbarium. We found plenty in
Angas Inlet from the Postal Institute’s boat ramp, to the end of the
Fishermen’s jetty (east of the public boatramp).
I have
found Bugula neritina growing on an old Caulerpa racemosa ramulus,
and three young colonies of an encrusting bryozoan (probably Celleporaria
cristata; see Gordon D. P. 1989 p33 and plate 16, D-F) on a Caulerpa
racemosa stolon. An older colony of what was probably the same encrusting bryozoan, was found on sea lettuce (Ulva sp.). Shepherd and
Thomas (1982) Plate 28,4 is of a mature live colony.
So, like
the European feather-duster worm, Caulerpa racemosa provides a stable
substrate for other marine benthic organisms in a zone otherwise denuded of
marine macrophytes (perhaps because of high nitrogen levels; see Neverauskas
1988; and presentations at the Friends of Gulf St Vincent forum held at SARDI
Hamra Road on 22/10/05).
Close examination of a specimen of Caulerpa
taxifolia from Angas Inlet (8/8/05) revealed the presence of many different
phyla of marine invertebrates. Terebellids and other segmented worms,
Amphipods, a brittle star, a seasquirt on a stolon, numerous silt tubes
probably inhabited by crustaceans (Amphipods) and Isopods. The angle between
main fronds and branches provided a microhabitat for many of the silt tubes.
Rhizoids looked as if they would also provide a microhabitat for invertebrates
(and many microorganisms). A knee-deep crop of C. taxifolia is likely to
be far more productive than a “bare” area on which marine flowering plants have
been killed off by other factors in a polluted environment. I say “bare”
because I am well aware that there are microscopic photosynthetic and
chemosynthetic organisms that can fix a lot of energy in these areas. I cannot
comment on whether C. taxifolia can invade a healthy area of marine
flowering plants not debilitated by polluting factors. We might be lucky to
have C. taxifolia, just as many of our Arid Zone graziers feel lucky to
have weeds like Barley Grass, Salvation Jane and Wards Weed (Carrichtera
annua) stabilizing soils long-since denuded of native species by
overgrazing. It is time to look at positive aspects of C. taxifolia.

Picture 6
C. racemosa recently
found in the Port River and Angas Inlet
REFERENCES
Brock B.
J. (1979) Biology of Bryozoa Involved in Fouling at Outer Harbour and Angas
Inlet. Unpublished M.Sc. Thesis University of Adelaide
Zoology Dept.
Brock B.
J. (1985) South Australian fouling Bryozoans. In Nielsen C.
and Larwood G. P. (Eds.) Bryozoa: Ordovicion to
Recent. (Olsen & Olsen) p.p. 45-49.
Gordon D.
P. (1989) The Marine Fauna of New Zealand: Bryozoa:
Gymnolaemata (Cheilostomata Ascophorina)
from the Western South Island Continental Shelf and Slope. New Zealand
Oceanographic Institute Memoir 95 plate 16, D.E.F., Celleporaria cristata.
Lucas A.
H. S. (1936) The Seaweeds of South Australia. Govt. Printer Adelaide.
Neverauskas V. P. (1988) Accumulation of periphyton on artificial substrata
near sewage sludge outfalls at Glenelg and Port Adelaide, South Australia. Trans.
R. Soc. S.Aust. 112 (4) p.p. 175-177.
Shepherd
S. A. and Thomas I. M. (1982) Marine Invertebrates of Southern Australia Part 1
Plates 15,3 and 15,4 for Cassiopea ndrosia, and
28,4 for mature live Celleporaria cristata.
Shepherd
S. A. and Womersley H. B. S. (1970) The sublittoral
ecology of West Island, South Australia 1. Environmental
Features and the Algal Ecology. Trans. R. Soc. S.
Aust. 94 p.p. 105-138.
Shepherd
S. A. and Womersley H. B. S. (1971) Pearson Island Expedition 1969.-7. The Subtidal Ecology of Benthic Algae. Trans.
R. Soc. S. Aust. 95 (3) p.p.155-167.
The other
parts were: 1. Narrative 2. Geomorphology 3. Contributions to the Land Flora 4. The
Pearson Island Wallaby 5. Reptiles 6. Birds 7. Helminths
Shepherd
S. A. and Womersley H. B. S. (1976) The subtidal algal
and seagrass ecology of St Francis Island, South Australia. Trans.
R. Soc. S. Aust. 100 (4) p.p.177-191.
Thomas I.
M. et al (1986) The effects of cooling water discharge
on the intertidal fauna in the Port River estuary, South Australia. Trans. R.
Soc. S. Aust. 110 (4) p.p. 159-172.
Womersley H. B. S. (1984) The Marine Benthic Flora of Southern Australia. Part 1. (Govt. Printer, Adelaide). Caulerpa spp., p.p. 253-274.
Preliminary Field Observations of Mating and Spawning
in the Squid Sepioteuthis australis
TROY
M. JANTZEN* AND JON N. HAVENHAND#
School
of Biological Sciences, Flinders University,
GPO
Box 2100, Adelaide, South Australia, 5001
*
To whom correspondence should be addressed. E-mail: Troy.Jantzen@
bms.com
#
Current address: Tjämö Marine Biological Laboratory, Göteborg University, 452 96 Strömstad,
Sweden.
Received
15 February 2002; accepted 31 January 2003.
Reference:
Biol. Bul. 204:290 - 304 (June 2003)
C 2003
Marine Biological Laboratory
Sepioteuthis australis is a moderately large (≈30 cm mantle length) teuthoid squid, common throughout the coastal waters of
southern Australia and New Zealand (Mangold and
Clarke, 1998). Although prevalent throughout this region, no observations of
copulation have been reported for this species, and spawning behavior has been
reported only once in situ [as S. bilineata (Larcombe and Russell, 1971, see Mangold and Clarke, 1998)].
The object of the present note is to contribute to the knowledge of mating
and spawning behavior of S. australis. The observations
described here are the result of preliminary observations at the latter stages
of the spawning period of this little-studied species. We provide a brief
overview of S. australis mating and spawning behavior in the field, together
with an insight into the proposed research that will be conducted on this
species in future spawning seasons.
Materials and Methods
Throughout summer, S. australis move inshore to spawn, frequently
utilizing the same spawning grounds each year (unpubl.
observ.). Observations of S. australis reported
here were obtained from a spawning ground approximately 20-40 m offshore (from
the low tide mark) from the suburb of Marino, South Australia (130°30'E,
38°02'S). The benthos in this area consists of seagrass (Amphibolis
antarctica) and brown
macroalgae (Sargassum spp.) interspersed with
patches of bare sand and rock, and extends for several kilometers along the
coastline in this manner. The site at Marino is one of several spawning sites
for S. australis along this stretch of coastline, all of which share
these benthic characteristics.
During the spawning season, S. australis lay eggs in clusters
consisting of numerous egg capsules (approximately 300 capsules per cluster;
Larcombe and Russell, 1971), with each capsule containing 3-6 eggs. These egg
clusters are commonly found attached to the thalli of
the brown macroalgae Sargassum spp. This genus
of macroalgae is also used as an egg deposition site for other squid species
such as S. lessoniana (Segawa, 1987).
A total of nearly 4 h of ad libitum sampling
(Martin and Bateson, 1993) were made over a 3 d
period by SCUBA and snorkeling. Water depth on the spawning ground varied
between 2-4 m depending on the tide. Squid behavior throughout the observation
period was photographed with a Nikonos V camera. Attempts to record mating and
spawning behavior using a Canon E850-Hi video camera in waterproof housing were
unsuccessful due to an error with the tracking system on the camera. As a
result, valuable ‘one-zero’ sampling (Martin and Bateson,
1993) data of mating and spawning could be analyzed. Furthermore, body
patterning of the adults with each behavior could not be classified.
Results and Discussion
Egg Laying and Agonistic Behavior.—Larcombe and
Russell (1971) documented the behavior of six adult S. australis (two
male, four female) as the females attached egg capsules to an egg cluster that
had detached from its normal habitat and had become entangled in an artificial
reef. In contrast, the observations reported here describe spawning by
numerous individuals on egg clusters attached to Sargassum spp.
Sex ratio on the spawning ground was male biased. Although quantitative
measurements of the specific number of males and females in view at any one
time were not obtainable from the video footage, direct observation showed that
all females were paired with a male while several unpaired males swam amongst
the paired individuals. These unpaired males were observed attempting to
displace paired males from their mate: a behavior that was met with fierce
agonistic responses from the paired male who would endeavor to remain between
the female and the competitor using his mantle and fins. Agonistic behavior
often escalated into a fight with both males extending their arms and tentacles
and colliding into each other (Fig. 1). If the unpaired male did not withdraw,
a swimming fight would result, and the two males rapidly swam out of visible
range while continually colliding in this manner. ‘Swimming fights’ left the
female unattended and subject to pairing with other competitive males. Although
this behavior appears detrimental to the paired male, it may be advantageous to
the female, and a similar behavior has been observed in the cuttlefish Sepia
apama where females engage in extra pair copulations while a guarding male
is preoccupied with fighting with a competitor (R. T. Hanlon pers. comm.,
2000).
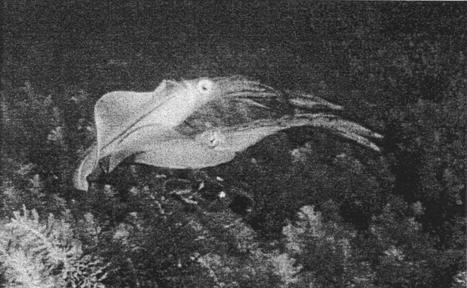
Figure 1. Two males fighting. The paired male (bottom) is
using his body
and fins to
‘guard’ his mate (background) from the advances of an
unpaired male (top).
In one instance, rather than a ‘swimming fight’, agonistic behavior
escalated to physical combat (DiMarco and Hanlon, 1997) and a paired male was
seen wrapping his tentacles and arms completely around the mid-mantle region
of an unpaired male. This behavior has also previously been observed in fights
among S. apama and is assumed to be an attempt to bite the intruder (R.
T. Hanlon pers. comm., 2000). Like the response of the unpaired males in the S.
apama fights, the unpaired S. australis male immediately jetted
away.
As well as defending their female from other males by fighting, paired
males guarded the female while she laid eggs. ‘Mate-guarding’ (sensu Sauer et al., 1992; Hanlon and Messenger, 1996)
involved the male remaining a few centimeters above his mate while she attached
egg capsules to a cluster (Fig. 2). Immediately following egg-laying, the pair
moved backwards away from the egg cluster and began a ‘mutual-rocking’ behavior
(Moynihan and Rodaniche, 1982; Hanlon and Messenger, 1996) in which the pair
swam side-by-side in a slow and deliberate forward and back motion. This
behavior stopped when the female approached the egg cluster and deposited a new
capsule amongst it in the same manner as that described by Larcombe and Russell
(1971).
 guarding a female.jpg)
Figure 2. Male (top) guarding a female (below) as she prepares to add
a new egg
capsule to a cluster (not visible in photo). Broken
spermatophores from a previous mating attempt (successful mating
results in spermatophores being deposited in the buccal region of the
receptive female) can be seen on the dorsal surface of the female.
Egg laying behavior of squid in spawning aggregations has been observed in
numerous species congregating around a large, central, relatively exposed,
communal egg cluster (Griswold and Prezioso, 1981; Sauer et al., 1992; Segawa
et al., 1993; Hanlon et al, 1994; 1997; Hanlon and Messenger, 1996). In our
observations of S. australis spawning, no more than three paired females
were seen laying eggs in the same egg cluster. Furthermore, although common in
some squid species (Hanlon and Messenger, 1996), no distinctive ‘zones’ of
mating and spawning activity could be identified for S. australis throughout
the observational period. It is possible that further observation will clarify
this issue.
Copulation Behavior of Paired Males.—Copulation in
Sepioteuthis involves the male depositing spermatophores into either the mantle
cavity when the male approaches from below the receptive female (Segawa, 1987;
Segawa et al., 1993), or onto the arm region when the
male approaches from beside or above the female (Moynihan and Rodaniche, 1982;
Boal and Gonzalez, 1998). When spermatophores are placed on the female arm
region, the male does so in one of two ways: in reports of S. sepioidea mating
in the field, the male positions himself next to, or ahead of the female, turns
towards her, and ‘strikes’ the female on the ‘forehead’. Alternatively, reports
of S. lessoniana mating in the laboratory describe a mating position in
which the male ‘flips’ upside-down to deposit spermatophores on the head or
arms of the female (Boal and Gonzalez, 1998). It is this latter ‘male-upturned’
mating position that was observed by paired males of S. australis in the
field.
Mating with the male in the upturned position was observed on six
occasions, each of which occurred prior to egg laying by the female while the
spawning pair was in close proximity to the egg cluster (usually <1.5 m).
Boal and Gonzalez (1998) categorized the male-upturned mating behavior of S.
lessoniana into four distinct behaviors, of which only three were observed
here: ‘pre-mating behavior’, ‘flip’, and ‘contact’; the fourth (‘attempt’) not
being observed in S. australis.
S. australis still displayed some behavioral traits consistent with S.
sepioidea mating in the field (Moynihan and Rodaniche, 1982). Unlike
‘pre-mating’ behavior in S. lessoniana that involved rapid back and
forth swimming by the mating pair (Boal and Gonzalez, 1998), ‘mutual-rocking’
in S. australis was slow and deliberate, similar to that described for S.
sepioidea (Moynihan and Rodaniche, 1982). Immediately prior to mating, male
S. australis were observed to fold back one tentacle horizontally toward
the female for a few seconds, such that the suckers on the club of the male
faced toward the female, a behavior that may be unique to S. australis mating.
The purpose of this ‘club display’ behavior remains unclear,
however it may signal the male's intention to mate with the female.
Following the male ‘club display’ to the female, the male was observed to
move directly above, or slightly anterior to, the body
of the female. The male then swiftly rotated 180° around the longitudinal axis
to achieve an inverted position above the female. This ‘flip’ action was
observed in males positioned both alongside as well as above the female in S.
lessoniana (Boal and Gonzalez, 1998). However, in S. australis it
occurred when the male was above the female.
While inverted, the male reached down with an arm and made cont with the
buccal area of the female (Fig. 3). From our observations we were unable to
determine if mating in this manner was successful. Unlike S. lessoniana in
which mating ended with the female rapidly jetting away (Boal and Gonzalez,
1998), ‘contact’ in S. australis was terminated when the male returned
to his original position alongside the female, similar to the post-mating
behavior described for S. sepioidea (Moynihan and Rodaniche, 1982). In S.
sepioidea spermatophores are visible on the hectocotlyus of the male prior
to mating (Arnold, 1965). This was not the case for the mating behavior
described here and the only spermatophores observed were those affixed to the
dorsal mantle of a female (Fig. 2). It is assumed that a male placed these spermatophores
there in an unsuccessful mating attempt, although the behavior that resulted in
that placement was not observed.

Figure 3. Lateral view of the male upturned mating position. The male
(top) can be seen in an upside down position
reaching down in an attempt
to deposit
spermatophores in the buccal region of the female (below).
Extra-pair Copulations.—As well as ‘male-upturned’ mating by paired males, behavior
consistent with extra-pair copulations in other species was observed by
unpaired S. australis males. In Loligo
pealei and L. vulgaris reynaudii,
extra-pair copulations occur by ‘sneaker males’ mating with females both at the
large communal egg mass, and at a short distance from the egg mass (the
‘mating/sneaker zone’; Hanlon, 1996; Hanlon and Messenger, 1996; Hanlon et al.,
in press). All of the extra-pair copulations that were observed in S.
australis occurred as a female added a new egg capsule to an egg cluster.
While laying eggs, the depth of the algal canopy was such that only the
mantle tip of the female protruded above the algae. Therefore, males guarding a
mate were always positioned some distance above the substratum either
immediately above or beside the female. Unpaired males were observed to swim
rapidly toward the female from the side or behind, dart amongst the Sargassum
and make physical contact with the female before quickly jetting away. The
nature of this contact was similar to the ‘sneaker’ mating observed in L.
pealei (Hanlon et al., 1994).
‘Sneaker’ mating by an unpaired male was identified once throughout the
observational period, while at least two other encounters showed aspects of
sneaking mating. In the first instance, an unpaired male made contact with the
arms of a female which was visibly laying eggs in a cluster. This behavior
occurred very rapidly such that it was not possible to determine if mating was successful, and (if so) where the spermatophores were
deposited. On two further occasions a male was seen disappearing into the algal
canopy within 0.5 m of a female laying eggs, soon
afterward reappearing and quickly jetting away. In these cases the head of the
female was almost completely obscured from view by the Sargassum and no
copulation was seen. However, behavior of the unpaired male in these latter two
instances was identical to that seen in the previously described encounter.

Figure 4. Unpaired male (left) attempting to displace another male (middle)
from his mate (right). The two males have tentacles and arms out-stretched
in a sign
of agonistic behavior. In the bottom left comer, an empty egg
capsule is noticeable.
Empty Egg Capsules.—Egg capsules were attached to the top of the Sargassum in
the spawning area on each day of observation (Fig. 4). Closer examination
revealed that these egg capsules did not contain any eggs. Furthermore, these
capsules were absent in the week following the spawning event (although
numerous egg clusters remained present). The purpose of these empty capsules is
not clear, however at least two possible functions can be speculated: (1)
locational ‘markers’: given our observations of repeated egg laying on
established egg clusters, the migration to and from these clusters during the
‘mutual-rocking’, and the dense algal canopy which frequently obscured egg
clusters from view, these empty capsules may flag the position of egg clusters,
facilitating their location by adults. (2) Capsules act as spawning stimulants:
as egg capsules have been demonstrated to induce male agonistic behaviour in
laboratory trials of L. pealeii (King et al.,
1999), and promote spawning in L. pealii (Arnold, 1962) and Loligo opalescens (Hurley,
1977), egg capsules (empty or full) may stimulate spawning of adults entering
the area. The reason for this may be visual or, as recent studies have suggested,
may be a response to a chemical or tactile stimulus embedded in the egg
capsules (King et al., 1999). Further studies are required to verify these
hypotheses.
conclusions
Observations of a natural spawning aggregation of
approximately 40 S. australis in South Australia have revealed several
previously undescribed aspects of spawning behavior
in this species. Male S. australis repeatedly copulated in the
‘male-upturned’ position and engage in behavior similar to extra-pair
copulations that are reported for other squid species. Further research is
being conducted in an attempt to determine the precise nature of these
behavioral traits. Focal animal sampling of fighting, mating and spawning
behavior using a digital camera will enable specific body patterning of each
sex to be analyzed. Continuous recording of the focal animal(s) will also
identify duration of spawning and mating, time between these events, and the
frequency and characteristics of extra-pair copulations. In addition, empty egg
capsules, which may serve as locational markers and/or spawning stimulants,
were observed deposited on the distal fronds of macroalgae within the spawning
area Laboratory and field studies will investigate this unusual behavior.
acknowledgments
We are thankful to L. van Camp for comments on this
research, and to B. Lock, A. Mack, and A. Hirst for
help SCUBA diving and snorkeling in the field.
literature cited
Arnold, J. M. 1962. Mating behavior and social structure
in Loligo pealii. Biol. Bull.
123: 53-57.
______. 1965. Observations on the mating behavior of the squid Sepioteuthis
sepioidea. Bull. Mar.Sci. 15:216-222.
Boal, J. G. and S. A. Gonzalez. 1998.
Social behaviour of individual oval squids (Cephalopoda,
Teuthoidea, Loliginidae,
Sepioteuthis lessoniana) within a captive school. Ethology
104: 161-178.
DiMarco, F. P. and R. T. Hanlon. 1997.
Agonistic behavior in the squid Loligo plei (Loliginidae, Teuthoidea): Fighting tactics and the effects of size and
resource value. Ethology 103: 89-108.
Griswold, C. A. and J. Prezioso. 1981. In
situ observations on reproductive behaviour of the long-finned squid, Loligo pealei. Fish.
Bull. U.S. 78: 945-947.
Hanlon, R.T., M. J. Smale and
W. H. H. Sauer. (in press). The mating system
of the squid Loligo vulgaris
reynaudii (Cephalopoda,
Mollusca) off South Africa: fighting, guarding,
sneaking, mating and egg laying behavior. Bull. Mar. Sci.
_______. 1996. Evolutionary games that squids play: Fighting, courting,
sneaking, and mating
behaviors
used for sexual selection in Loligo pealei.
Biol. Bull. 191: 309-310.
_______ and J. B. Messenger. 1996. Cephalopod
behaviour. Cambridge Univ. Press. Cambridge.
______, M. J. Smale and W. H.
H. Sauer. 1994. An ethogram of body
patterning behavior in the squid Loligo vulgaris reynaudii on the spawning
grounds in South Africa. Biol. Bull. 187:363-372.
______, M. R. Maxwell and N. Shashar. 1997. Behavioral dynamics that
would lead to multiple paternity within egg capsules of the squid Loligo pealei. Biol. Bull. 193: 212-214.
Hurley, A. C. 1977. Mating behavior of
the squid Loligo opalescens.
Mar. Behav. Physiol. 4:
195-203.
King, A. J., S. A. Adamo and R. T. Hanlon. 1999. Contact
with squid egg capsules increases agonistic behavior in male squid (Loligo pealeii). Biol. Bull.
197: 256-258.
Larcombe, M. F. and B. C. Russell. 1971.
Egg laying behaviour of the broad squid, Sepioteuthis bilineata.
NZ J. Mar. Freshw. Res. 5:3-11.
Mangold, K. and M. R. Clarke. 1998. Subclass Coleoidea. Pages 499-563 in P. L. Beesley, G.
J. B. Ross, and A. Wells, eds. Mollusca: The southern
synthesis. Fauna of Australia. CSIRO Publishing,
Melbourne.
Martin, P. and P. Bateson. 1993. Measuring Behaviour: An
Introductory Guide. Cambridge Univ. Press, Cambridge.
Moynihan, M. and A. F. Rodaniche. 1982.
The behavior and natural history of the Carribean
reef squid Sepioteuthis sepioidea. With a
consideration of social, signal, and defensive patterns for difficult and
dangerous environments. Adv. Ethology 25:
1-151.
Sauer, W. H. H., M. J. Smale, and M. R. Lipinski. 1992. The
location of spawning grounds, spawning and schooling behaviour of the squid Loligo vulgaris reynaudii (Cephalopoda: Myopsida) off the Eastern Cape Coast, South Africa. Mar.
Biol. 114: 97-107.
Segawa, S. 1987. Life history of the
oval squid, Sepioteuthis lessoniana in Kominato
and adjacent waters central Honshu, Japan. J. Tokyo Fish. 74: 67-105.
_____, T. Izuka,
T. Tamashiro, and T. Okutani. 1993. A
note on mating and egg deposition by Sepioteuthis lessoniana in Ishigaki Island, Okinawa, Southwestern Japan. Venus (Jap.
J. Malac.) 52:101-108.
addresses: (corresponding author): (T.M.J.) Marine Biology, School of Biological
Sciences, Flinders University, GPO Box 2100, Adelaide, South Australia, 5001. Tel. +618 8201 5184. Fax + 61 8 8201 3015.
E-mail: <Troy.Jantzen@flinders.edu.au>. (1,N.H.) Deputy Head, School
of Biological Sciences, Flinders University, GPOBox2100, Adelaide, South
Australia, 5001. Tel. +618 8201 2007. Fax+ 61 8 8201 3015. E-mail: <Jon.Havenhand@flinders.edu.au>.
by Steve Reynolds
Since the
publication of my article “Numerous Nudibranch Findings” in our June 2005
Newsletter (No.322), SA diver Dennis Hutson continues to make more nudibranch
discoveries.
Since then Dennis has found nudibranchs such as Noumea haliclona, Thecacera
pennigera, Noumea closei, Discodoris concinna, Dendrodoris (?nigra)
and Hypselodoris saintvincentius. These discoveries are discussed later,
but let’s go back to some details about those nudibranchs discussed in the first article.
“Numerous
Nudibranch Findings” reported findings of Polycera
hedgpethi, Elysia Sp.
(E.?expansa), Flabellina Spp. (3)
and Aegires villosus by Dennis.
Each of
these species features in “1001 Nudibranchs” by Neville Coleman. Polycera hedgpethi, however,
is incorrectly spelled as Polycera hedgepethi in that book. (I, myself, spelled ‘opisthobranch’ incorrectly in my article.)
Our
Society purchased “1001 Nudibranchs” since my article was written. It is
primarily used for identification and classification work for our Photo Index
but it is available from our library for limited loan to Society members (mlssa
No.1050).
“1001
Nudibranchs” does not include SA in the distribution details given for Polycera hedgpethi, Elysia Sp. (E.?expansa)*,
Flabellina Spp. (3)
and Aegires villosus.
This means that Dennis’s information is quite important.
*It may
not have been made entirely clear in my article “Numerous Nudibranch Findings”,
but Elysia species
Elysia Sp. are
not nudibranchs at all. They are, in fact, sap sucking slugs (Order Sacoglossa).
“The Sea Slug Forum – Species List” gives the
following details for Elysia species: -
Order Sacoglossa, Superfamily Elysioidea, Family Elysiidae.

Elysia species
On 8th
June 2005 Dennis sent an email message to Nerida
Wilson (formerly of the SA Museum) to let her know that specimens of Polycera hedgpethi
had returned to the North Haven boat ramp. Nerida
is now working at the Department of Biological Sciences at Auburn University in
Alabama, USA. Dennis had been looking out for Polycera
hedgpethi every week and discovered
them again whilst collecting water for his aquarium. He told Nerida that the size of the animals was small, about 10mm.
“If you know their growth rate you should be able to work out when they
actually returned. They’re the first I’ve seen this year. The water temperature
has now dropped to 16 degrees C.”
Polycera hedgpethi
I later
asked Nerida if she had received Dennis Hutson's message about the late reappearance of Polycera hedgpethi.
She replied that she had and that she had managed to incorporate it into her
paper before it went to the printers.
“Just in
time!” she said. “It’s a very interesting observation. The name honours Joel Hedgpethi, a marine scientist that worked mostly in
California, I think...here you go, a quick web search found this...
“Joel
Walker Hedgpeth, 1911-, Californian pycnogonid taxonomist and ecologist, active between the
1930s-1980s [Hedgpethia turpaeva,
1973, Coboldus hedgpethi
(J.L. Barnard 1969), Laophontodes hedgpethi Lang, 1965, Chaetozone
hedgpethi Blake, 1996, Polycera
hedgpethi Marcus, 1964, Ammothea
hedgpethi, Elysia hedgpethi ].”
“The Sea
Slug Forum – Species List” gives the following details for Polycera
hedgpethi :-
Order Nudibranchia, Suborder Doridina,
Family Polyceridae, Subfamily Polycerinae.
On 12th
February 2005 Dennis found a tiny 5 mm long nudibranch in Gulf St Vincent on
Seacliff Beach. As usual, Dennis took a photo of the tiny critter and sent it
in to the Sea Slug Forum at http://www.seaslugforum.net
asking for it to be identified.

Polycera hedgpethi
Noumea haliclona
The Sea
Slug Forum is operated by Dr Bill Rudman, Curator of
Molluscs at the Australian Museum.
In his
response to Dennis, Dr Bill Rudman said: -
“Dear
Dennis,
This is
interesting on two counts. Firstly, I think it is a juvenile of Noumea haliclona.
This is interesting because, although it is known from New South Wales,
Victoria and Tasmania, this is the first record I know of it from South
Australia. As you will see from the Fact Sheet, its colour matches one of the
colour forms found in Tasmania. The second interesting point is that its rhinophores are fused. Instead of being a pair, there
is one large central rhinophore apparently in a
single large pocket. This is obviously a result of some fault during its
larval development. If you are interested, have a look at the fact sheet
where I have listed other developmental abnormalities”.
The fact
sheet included these details: - “This small chromodorid
is common in south eastern Australia both intertidally
and sublittorally from northern New South Wales, to
Victoria and Tasmania. It is one of the red-spotted colour group of chromodorids from this region and exhibits both colour
variation within a region [sympatric variation] & distinctive regional
colour forms [allopatric variation], which are
illustrated on this page. There are no distinguishing anatomical features
between the regional colour morphs. Externally, small scattered red or orange
spots, and a red or orange mark on the front of the rhinophore
club are the two characters which link these forms together. They feed on a
range of pink and yellow aplysillid sponges.
Best
wishes,
Bill Rudman”
According
to the fact sheet, Noumea haliclona (Order Nudibranchia)
is of the Suborder Doridina, Superfamily Eudoridoidea
and Family Chromodorididae.
The fact
sheet can be seen at: -
http://www.seaslugforum.net/factsheet.cfm?base=noumhali1
. Noumea haliclona
features on page 83 of “1001 Nudibranchs”.
On 18th
June 2005 Dennis was diving solo at Outer Harbor when he discovered another
tiny (12mm) nudibranch on some Caulerpa (racemosa?). Dennis took this
photograph of the colourful little nudibranch and later sent it to me for
identification.
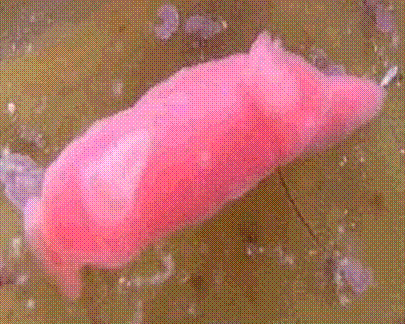
Noumea haliclona
Thecacera pennigera
I was
able to identify it from Neville Coleman’s book “Nudibranchs of the South
Pacific” as being Thecacera pennigera. I still suggested to Dennis that he send his
photo in to the Sea Slug Forum. He did that on the 20th June, with
the following message: -
“Can you
please confirm this is Thecacera pennigera?
Locality:
Outer Harbor, Adelaide, South Australia. Depth: 3 metres. Length: 12 mm. 18
June 2005. silty. Photographer:
Dennis Hutson.
Is it
native to Adelaide, South Australia?
Cheers,
Dennis Hutson”
Dennis
soon received the following reply from Dr Bill Rudman:
-
“Dear
Dennis,
Yes this
is Thecacera pennigera.
Your question about where it comes from is quite interesting. It was originally
described from England so we have assumed that it is a North Atlantic species.
However it has since been found in many parts of the world as you will see from
the Fact Sheet and accompanying messages. It feeds on bryozoans which are part
of the 'fouling community', a name for plants and animals which grow on
hard surfaces, and in particular, the bottom of boats. We have assumed
that this species has spread from the North Atlantic on the bottom of boats.
However most species of Thecacera are found in
the Indo-West Pacific region, and as you will see from
looking at the photos on the Forum, there is much more colour variation in
animals from the Pacific than from the North Atlantic. This could suggest that
the Pacific is the original home of this species and what we now find in the
North Atlantic is a population based on a few Pacific animals with small spots
that were transported there on the bottom of sailing ships two centuries ago.
I guess
this could be tested by DNA analysis.
Best
wishes,
Bill Rudman”
(These
details can be found at http://www.seaslugforum.net/display.cfm?id=14077
.)
The fact
sheet accompanying Bill’s reply indicated that Thecacera
pennigera (Order Nudibranchia)
belongs to the Suborder Doridina, Family Polyceridae and Subfamily Polycerinae*.
It said that the species had been originally reported from the Atlantic coast
of Europe, but it is now known from South Africa, West Africa, Pakistan, Japan,
Brazil, eastern Australia and New Zealand.
The fact
sheet can be seen at :-
http://www.seaslugforum.net/factsheet.cfm?base=thecpen
.
*This
makes Thecacera pennigera
a close relative of Polycera hedgpethi which shares the same details.
“1001
Nudibranchs” features both Thecacera pennigera and Noumea haliclona, but the book does not include SA in the
distribution details given for either. Once more, this means that Dennis’s
information is quite important.
Thecacera pennigera is featured on page 47 of “1001 Nudibranchs” and page 14
of “Nudibranchs of the South Pacific”.

Thecacera pennigera
Again, as
with Polycera hedgpethi,
CRIMP’s “A Guide to the Introduced Marine Species in
Australian Waters” by Dianne M Furlani has a page of
details about Thecacera pennigera
under “Mollusca” (updated May 1996). (CRIMP is
the acronym for Centre for Research on Introduced Marine Pests.) Also as with Polycera hedgpethi,
this page also features a Richard Willan and Neville
Coleman 1984 photo of the nudibranch. This appears to be one of the same photos
(AMPI 73) featured in “1001 Nudibranchs” and the same one featured in
“Nudibranchs of the South Pacific Vol.1” by Neville Coleman.
(AMPI is
the Australasian Marine Photographic Index which is curated
by Neville Coleman. According to Neville, the Index is “a comprehensive
scientifically curated visual identification system
containing over 150,000 individual transparencies covering almost every aspect
of aquatic natural history”. The colour transparencies of living animals and
plants in the Index are “cross-referenced against identified specimens housed
in museums and scientific institutions”.)
“A Guide
to the Introduced Marine Species in Australian Waters” says that, although Thecacera pennigera is
a Polycerid, it “differs consistently from Polycera in (both) absence of velar process and presence of
rhinophore sheath”. Also, “body
tapers to long foot, 2 lateral extensions posteriorly, and no velar process”.
According
to the glossary in both “1001 Nudibranchs” and “Nudibranchs of the South
Pacific Vol.1”, a ‘rhinophore (rhinophoral)
sheath’ is an “upstanding flange from the antero-lateral
part of the mantle (of dendronotacean nudibranchs)
into which the rhinophore can be contracted”. The
glossary says that a ‘rhinophore’ is “sensory
tentacle on the head or anterior section of the mantle of opisthobranchs”.
I also
wanted to know what ‘velar’ was. I couldn’t find the word in any of the
glossaries that I checked out. It was, however, shown in a diagram of a dorid nudibranch in “1001 Nudibranchs”. There also seemed
to be a connection between ‘velar’ and ‘velum’. It so happened that I had the
opportunity to ask Greg Rouse at the SA Museum about this matter.
“Do you
know much about nudibranchs?” I asked him. “Can you explain what velar/velum
are?”. Greg suggested that his partner, in the USA
(who happens to be Nerida Wilson) would be the best
person to help me. He said that he had passed my query on to her.
Nerida soon replied to my query, saying that “The velum is the
part of a nudibranch larva that is covered with cilia that help it swim/feed.
It has two (velar) lobes. There is a nice picture of a larvae where you can
clearly see the foot, and the velar lobes poking out from the shell on the sea
slug forum at :-
http://www.seaslugforum.net/factsheet.cfm?base=larvshel
.
I think
during metamorphosis that the velar lobes are reabsorbed into what becomes the
mantle. Hope it helps. Nerida”
I checked
out http://www.seaslugforum.net/factsheet.cfm?base=larvshel
where I found the following picture by Yoshi Hirano: -
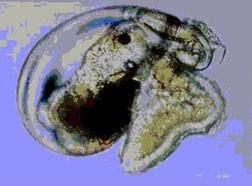
Yoshi Hirano's picture clearly
showing the transparent larval
shell of the aeolid nudibranch
Facelina bilineata.
This
would seem to be the picture referred to by Nerida
“of a larvae where you can clearly see the foot, and
the velar lobes poking out from the shell”.
Incidentally,
Neville Coleman gives most nudibranch species a common name. For example, he
calls Thecacera pennigera
“Winged Thecacera.” Noumea haliclona is called “Sponge Noumea”
and Aegires villosus
is called “Shaggy Aegires.” Polycera
hedgpethi is called “Hedgepeth’s
Polycera” (should be “Hedgpeth’s
Polycera”). The opisthobranch
Elysia expansa
is called “Wide Elysia.”
Dennis is
keeping his specimen of Elysia Sp. (E.?expansa) in his home
aquarium. The opisthobranch is doing very well in the
tank and it has laid eggs on a couple of occasions.
On 13th
August 2005 Dennis photographed a small specimen of the Sweet Ceratosoma,
Ceratosoma amoena at the Glenelg Tyre Reef.
Ceratosoma amoena
Ceratosoma amoena is featured on pages 64-5 of “1001 Nudibranchs” and page
39 of “Nudibranchs of the South Pacific”. According to “1001 Nudibranchs”, Ceratosoma
amoena (Order Nudibranchia)
belongs to the Family Chromdorididae.
There has
been some confusion over the correct scientific name for the Sweet Ceratosoma.
I have come across the name Ceratosoma amoenum
in place of Ceratosoma amoena. Nerida Wilson says that Ceratosoma amoena
is the correct name. She explains, however, that the species used to be placed
in the genus Chromodoris (as Chromodoris amoenum).
It was later moved into the genus Ceratosoma and the species name was then
changed to match the gender of the new genus (Ceratosoma amoena).
Later on,
Nerida explained the above details to me once more by
saying, “The animal was first called (Chromodoris) amoenum,
but when it was put in to the genus Ceratosoma instead, the specific name was
changed to amoena to be in agreement with the
gender of the genus name Ceratosoma”.
It seems
then that different genus are considered as different
gender. Nerida told me “It’s confusing to know which
genus name is feminine or masculine, etc, and when a change is needed. However,
while it is strictly correct to change the species name to match (the gender of
the genus name), not all people stick to this. But for your species, amoena is the correct and common usage nowadays” she
said.
On 10th
July 2005 Dennis sent me two more photos of nudibranchs that he had found on
the Glenelg Barge the day before. He also sent both of the photos on to Nerida Wilson, Thierry Laperousaz,
Senior Collection Manager for Marine Invertebrates at the SA Museum’s Science
Centre and the Sea Slug Forum. Dennis and I had several discussions about the
identification of the two nudibranchs but Nerida was
the first person to respond with an ID.
“I think
it is Noumea sulphurea”
she said, “although there is a possibility it is N.closei.
You may be able to see better whether there are any
small orange specks on the back, which will likely indicate N.closei”.
Dennis
also thought that it might be Noumea sulphurea. He directed me to http://www.seaslugforum.net/factsheet.cfm?base=noumsulp2
.
The web
page features two photos of Noumea sulphurea. They were both said to be the “Bass Strait
colour form” (both from Tasmania in 1985). The distribution for Noumea sulphurea was
given as being “Southern Australia - Tasmania, Victoria & South
Australia”.
The web
page went on to say that “Specimens from Tasmania, Victoria and South Australia
differ from New South Wales animals in having regularly spaced white specks all
over the dorsum except for a clear band near the edge, the specks apparently
being formed from aggregations of subepithelial
granules. In some specimens there is also an aggregation of these granules
right at the edge of the mantle to form a broken white line along the edge. The
colour of the orange spots is also much less brilliantly opaque than New South
Wales animals, the spots often being almost invisble
in smaller specimens.
This
‘Bass Strait colour form’ is very similar in colour to Noumea
closei and the yellow Tasmanian colour form of Noumea haliclona”.
Dennis’s
enquiry was posted to the Sea Slug Forum site on 20th July 2005 at http://www.seaslugforum.net/display.cfm?id=14265.
Bill Rudman said that it was, in fact, Noumea closei. The
web page read: -
“Noumea closei from
South Australia (From: Dennis Hutson)”

Ceratosoma amoena
Noumea species
Could
this please be identified and is it native to South Australian waters?
Locality:
wreck of the Barge, Glenelg, South Australia. Depth: 16 m.
Length: 10 mm. 9 July 2005, Deck of the wreck, Photographer: Dennis Hutson”.
Bill Rudman responded with: -
“Dear
Dennis,
This is Noumea closei, which
is known from Victoria, Tasmania and South Australia. Have a look at the Fact
Sheet for more information on it and the other similarly coloured species.
Best
wishes,
Bill Rudman”
The facts
sheets for Noumea closei
and Noumea sulphurea
are http://www.seaslugforum.net/noumclos.htm
and
http://www.seaslugforum.net/noumhali1.htm
respectively.
Dennis
told me that he thought that it was N.sulphurea
because of this other link - http://www.seaslugforum.net/display.cfm?id=6309
. “Bill mentions the white marks on top” said Dennis. The web page read: -
“Noumea closei from
South Australia
March 10,
2002
From:
Stuart Hutchison
Hi Bill,
Here’s
one were not sure about from the wreck of The South Australian,
Adelaide, South Australia on 26 Sep 1999. Depth 20m, length
15mm.
Regards,
Stuart.
Thanks
Stuart,
I am
pretty sure, from the white marks, that this is Noumea
closei. However if you look at the Bass Strait
colour form of Noumea sulphurea
you will see it is almost identical externally. There is an interesting story
attached to how I first realised there were two species involved. N. closei when preserved in formalin turns dark brown,
sometimes almost black, while Noumea sulphurea gradually loses all its colour, becoming
translucent white. One day, when I was choosing some specimens to dissect, I
decided I had better check some dark and some light coloured ones just in case
I had missed something. To my great surprise I found their radular
morphology was quite different.
Using the
preserved colour of a slug is not the most practical way to identify a species,
but as with species of Rostanga, sometimes
external shape and colour just isn't enough.
Best wishes,
Bill Rudman”
Noumea sulphurea is featured on page 84 of “1001 Nudibranchs” and page 42
of “Nudibranchs of the South Pacific”. Neville calls it a Sulphur Noumea. Noumea closei is not in either book.
We still
don’t know, at the time of writing, what species Dennis’s second photograph is.
Neither Nerida Wilson, Bill Rudman or Neville Coleman have been able to identify
it.

Noumea species
An aeolid nudibranch on the Barge at Glenelg
After
viewing MLSSA’s copy of “1001 Nudibranchs”, Dennis ordered a copy for himself to assist with identifications. He found that one of
Nerida Wilson’s nudibranch photos is featured on page
89. It is a Doriopsis
pectin (Blue Doriopsis).
Dennis
photographed specimens of Discodoris concinna and a Dendrodoris
whilst diving at Outer Harbor. He reported the discoveries to Thierry Laperousaz at the SA Museum.
Thierry
advised Dennis that he thought that Discodoris
concinna was rare in SA. Dennis has since
collected a specimen for the museum.

An aeolid nudibranch on the Barge at Glenelg
Discodoris concinna
Discodoris concinna is featured on page 23 of “Nudibranchs of the South
Pacific”. Thierry thought that the Dendrodoris that
Dennis had photographed was Dendrodoris nigra. Dennis feels that it is actually Dendrodoris fumata
as it is more commonly found in our waters. He says that there is a subtle
difference in the gill structure but they are very difficult to tell apart.

Discodoris concinna
Dendrodoris (?nigra)
Stuart
Hutchinson thought that he had taken some photos of Dendrodoris
nigra at Edithburgh jetty in January 1999. He
sent two photos to the Sea Slug Forum in February 2000. Bill Rudman responded as follows: -
“Dear
Stuart,
This is Dendrodoris fumata,
not Dendrodoris nigra.
Have a look at my comments on each page to see the differences ( http://www.sealugforum.net/dendfuma.htm
and http://www.seaslugforum.net/dendnigr.htm
).
These two
species have been confusing us for over a century as both have the same range
of colour forms. One good difference seems to be the shape and number of gills.
In D. fumata there are a few (usually 5) large
gills, which are usually extended out to the mantle edge. In D. nigra there are many small gills arranged in a
cup-shaped circle. In both your photos I think I can see parts of the bright
yellow egg ribbon. It also appears that the sponge they are on is a food
source.
Best
wishes,
Bill Rudman”
These
details, with the two photos, are available at :-
http://www.seaslugforum.net/display.cfm?id=1818
.
Dendrodoris fumata is featured on page 86 of “1001 Nudibranchs” and Dendrodoris nigra is
featured on page 87. Neville Coleman calls Dendrodoris
fumata Hazy Dendrodoris and
Dendrodoris nigra
Black Dendrodoris. “1001 Nudibranchs” says that
both belong to the Dendrodorididae family.
Dennis
photographed a Hypselodoris species of nudibranch
whilst diving at the Blocks at Glenelg on 21st November 2004. Someone suggested
that it was Hypselodoris infucata but Dennis thought that it might be Hypselodoris saintvincentius
as this species is more commonly found in our waters. As he understands it,
the difference is in the colouring. On 26th
August 2005 Dennis posted the following details on to the Sea Slug Forum: -
“Could you please confirm the id of this critter? The photo was taken at a site
known as the Glenelg Blocks, which is located approx. 500m off the beach of
Glenelg, South Australia, Gulf St. Vincent. I was told it was Hypselodoris infucata
but I thought it might be Hypselodoris saintvincentius. I understand there is a slight
difference in colour. Thanks for your help.
.jpg)
Dendrodoris (?nigra)
Hypselodoris saintvincentius
Locality:
Glenelg Blocks off Glenelg Beach, South Australia, Southern Ocean. Depth: 5 m.
Length: 40 mm. 21 November 2004. Rocky bottom Photographer: Dennis Hutson”
Bill’s
reply was as follows: -
“Dear
Dennis,
Your
animal is Hypselodoris saintvincentius.
As I discuss in a recent message [http://www.seaslugforum.net/find.cfm?id=14285],
it is not that easy to separate from H. infucata.
Best
wishes,
Bill Rudman”
Hypselodoris infucata is featured on page 80 of “1001 Nudibranchs”. Neville
Coleman calls it Painted Hypselodoris. “1001
Nudibranchs” says that, like Noumea closei, Noumea sulphurea and Ceratosoma amoena,
Hypselodoris infucata
belongs to the Chromodorididae family.
In August
2005 Nerida asked Dennis and I to arrange for the
collection of Polycera hedgpethi
sspecimens for her. She said that she was trying
to set up a project that uses the DNA sequences of all P.hedgpethi
populations worldwide, to try and determine which populations are connected by
gene flow, etc.. “I am hoping to be able to determine where the natural range
of Polycera was in the world, and if it is truly
introduced or not. This requires many more specimens than Dennis collected for
me last year. Is there any chance that you might be able to help, or know
someone who may? I basically need about 25 specimens,
and someone to take them into the museum where Greg or Thierry can fix them for
me” she said.
That same
month (August 2005) Dennis and I dived at Outer Harbor in low visibility. We
couldn’t find any Polycera hedgpethi but Dennis managed to find another Elysia species. He also photographed and collected
another unusual nudibranch.

Hypselodoris saintvincentius
An unusual nudibranch
found at Outer Harbor
After the
dive Dennis studied the photograph and “1001 Nudibranchs”. He suggested in an
email message to me that it may possibly be a Discodoris
lilacina (p.56) or Dendrodoris
albobrunnea (p.86). Whilst “leaning towards the
first” he decided to post it onto the Sea Slug Forum for confirmation.
On Sunday
28th August I snorkeled at Dock 1 in the Port River to search for
specimens of Polycera hedgpethi
for Nerida. I didn’t have any luck at all and advised
Nerida and Dennis. Dennis reported that he had
collected 4 specimens for her from the North Haven boat ramp. This gave Dennis
the opportunity to take some more photos of the species. He said that he was
taking them in to the SA Museum alive for Thierry to fix for Nerida.

An unusual nudibranch found at Outer Harbor
Dennis
has set up his own web site which features different galleries of his photos,
both above and below water. The address for the site is :-
http://members.ozemail.com.au/~dghutson/
.
Many of
Dennis’s nudibranch photos are being posted to the site.
Many
thanks to Dennis Hutson, Nerida Wilson, Thierry Laperouzas and Bill Rudman for
their considerable assistance with the above details.
REFERENCES:
“Nudibranchs of
the South Pacific Vol.1” by Neville Coleman, Sea Australia Resource Centre,
1989, ISBN 0 947 325 02 6.
“1001
Nudibranchs” by Neville Coleman, Underwater Geographic P/L, 2001, ISBN
0947325255 – mlssa No.1050.
“Numerous
Nudibranch Findings” by Steve Reynolds, MLSSA Newsletter June 2005 (No.322).
Australasian Nudibranch News 2:4
(Volume 2 (4): 3, December 1999)
The Sea Slug Forum – Species List
PHOTO CREDITS
1. Dennis Hutson of Elysia species
2. Dennis Hutson of Polycera hedgpethi
3. Dennis Hutson of Noumea
haliclona
4. Dennis Hutson of Thecacera pennigera
5. Yoshi Hirano
of the transparent larval shell of the aeolid
nudibranch Facelina bilineata.
6. Dennis Hutson of Ceratosoma amoena
7. Dennis Hutson of Noumea closei
8. Dennis Hutson of an aeolid nudibranch found on the Barge at Glenelg
9. Dennis Hutson of Discodoris concinna
10. Dennis Hutson of the Dendrodoris (?nigra)
11. Dennis Hutson of Hypselodoris
saintvincentius
12. Dennis Hutson of an unusual nudibranch found at Outer
Harbor
Recent Requests to Our Society for Information
by Steve
Reynolds
Our Society occasionally receives
requests (usually by email) for information on marine topics. A few such
requests, and subsequent replies, have been put together below: -
Late in 2003 we received the
following request for information:-
“Hi… I’m a
Bachelor of Science student (Biology major) looking for some information on the
Leafy Seadragon for an ecology assignment. It’s only for my 2nd year
Ecology essay, but it’ll be invaluable. As my studies continue, I plan to focus
on the marine ecology of the Hallett Cove/Marino area where I live – a very
interesting and unique little bit of coastline. I’m actually a plant science
fanatic, so my special interest is actually the local seaweed!
Cheers,
Anissa Lato”
I was able to
provide Anissa with the references that she requested
for her assignment. I asked her to keep in touch and let us know how she gets
on with it.
The following message then came from
Heather Wadley in January 2005.
“Subject: I am in
search of information on the Leafy Sea Dragon.
Dear MLSSA
members,
My name is
Heather Wadley, I am a 12th grade student at a
Massachusetts school in the United States. I am taking an oceanography class,
and my teacher, Mrs. Streck is having us do a project
on a oceanic creature. I have looked online for the
internal and external structure of the Leafy Sea Dragon. I stumbled across your
newsletter when doing a search online, and saw that you offered the information
about the anatomy of a Leafy Sea Dragon, and I was wondering if it might be
possible for you to share the information, if you have pictures that are
possibly labeled, or not labeled. I greatly appreciate your help if you can
help me or not with the pictures. Again, thank you very much for your
assistance.
Sincerely,
Heather Wadley”
We sent the following reply to
Heather: -
“Hi Heather
Firstly, I have
attached two pictures from our Photo Index. You will find lots of articles
about the Leafy in our September 2004 newsletter on our web site. You will find
some more photos there. I have also attached a seadragon reference list that I
have compiled.
Here are some
details about Dragon Search’s Code of Conduct.
‘DIVING WITH
DRAGONS - CODE OF CONDUCT’
The ‘Diving with
Dragons - Code of Conduct’ sets out a few simple guidelines that divers can
follow to reduce their impact on seadragons. The “Diving with Dragons - Code of
Conduct” asks divers to: -
Leave seadragons
where they are;
Look but don't touch;
Respect their home range and avoid herding;
Avoid moving seadragons up or down in the water column;
Accept sea lice on seadragons in moderation;
Watch your feet and fins;
Take special care with male seadragons carrying eggs;
Turn the lights down when observing at night;
Clean up discarded fishing line found;
Dive right and watch your gear;
Respect the marine environment;
Remember fisheries regulations; and
Respect marine reserves.”
Hope that this
all helps.
Regards,
Steve Reynolds”
The following request came from
Robin Sharp in April 2005.
“Subject: Marine
Plan rare species Upper Spencer Gulf
Hi,
Appreciate if
you could email me back some information on the following species. Apparently
they are a relic species of interest within the Upper Spencer Gulf Marine plan.
I would like to obtain a photograph of these in order to better understand what
they actually are. Can you help?
1) A
flatworm (ancoratheca sp.)
(2) A
nudibranch (Discodoris sp.)
(3) An ophiuroid (Amphiura sp.)
Best Regards,
Robin Sharp,
Port Augusta”
We sent the following reply to
Robyn: -
“Hi Robyn
1) Flatworms
are Polyclads (Order Polycladida).
They belong to the Phylum Platyhelminthes. There is a
chapter (Chapter 5) about them in the book ”Marine
Invertebrates of Southern Australia – Part 1” edited by SA Shepherd & IM
Thomas. Ancoratheca australensis
is the first species described in the chapter. It is said to be recorded from
upper Spencer Gulf. The brief description says that the body is “oval, of firm
consistency, to about 10mm long. Dorsal surface light brown, dappled with dark
spots, except anteriorly and marginally where body is
whitish. Very small marginal eyes; minute eyes spread fanwise over cepahalic region to merge with marginal eyes”. A few
specimens of flatworms are pictured in the book – plates 22.4, 22.5, 22.6 23.1.
One book that specializes in Polyclads is “Marine
Flatworms, the World of Polyclads” (author unknown)
which is available for $39.95 (+ postage) from books@motpub.com.au .
2) Nudibranchs
are an Order of ophistobranchs (sea slugs) – Order Nudibranchia. Dorid nudibranchs
are a Family of nudibranchs – Family Dorididae. Discodorids are included in the Dorid
family. Three species of discodoris are pictured in
Neville Coleman’s book “”Nudibranchs of the South Pacific” (pages 22 –3). This
book is also available from books@motpub.com.au
at the price of $16.50.
3) Ophiuroids are brittlestars, a
class of starfish. Brittlestars belong to the Class Ophiuroidea.
The book ”Marine Invertebrates of Southern Australia-
part 1” edited by SA Shepherd & IM Thomas lists several species of Amphiura brittlestars (belonging
to the Family Amphiuridae) on page 437. Only two of
these species are described in the book – Amphiura constricta & Ophiocentrus pilosus (on page 425). Amphiura
are said to have “two squarish papillae at apex of
each jaw”. Amphiura constricta
is said to have “one erect distal oral papillae on each side of each jaw,
separated from the tooth papilla by a wide gap (Fig 10.15e on page 427). It is
said to be “A widespread and common species growing to 7mm disc diameter, with
arms up to 45mm long. The disc is fully scaled above and below, and the arms
bear 6-8 short spines on each segment. The tentacle pores are covered by a
single, large oval scale, and there is one distal oral papilla on each side of
the jaws. The radial shields are longer than wide, separated and divergent. The
disc is grey and the arms are banded light and dark. The species lives in algal
turf and amongst bryozoan and sponge rubble”.
Ophiocentrus pilosus is said to have “one or two squarish
apical oral papilla and one or two distal papillae” and “Disc scales bearing
simple short spinelets, no tentacle scales”
(Fig.10.15c on page 427). It is said to be “A large simple-armed brittlestar gowing to 18mm across
the disc with arms 140mm long. It is characterised by
having a Amphiura-type mouth
structure, with tall distal oral papilla on each side of the jaws, but it is
also completely lacks tentacle scales, and its disc is covered with small
scales and numerous short spinelets. It has up to 10
somewhat flat-tipped arm spines on each segment, and the lowermost are the
longest. A soft sediment dweller, this species is known mostly from SA to NSW”.
Our Photo Index
of SA Marine Life features a few photos of flatworms, dorid
nudibranchs and brittlestars. These photos can be
viewed on our web site at www.mlssa.asn.au
- flatworms on pages 16 & 17, dorid nudibranchs
on pages 1-3 and brittlestars on pages 8 & 9.
Much of the above
details are very involved but we trust that it helps a little.
Cheers
Steve Reynolds”
This request came from Des Fuller in
March 2005: -
“Hi.
I have been
trying to find out some info on the life cycle and growing time for Razor fish.
We collect them (to eat) each year on our holidays on Gulf St Vincent in
SA. But there are a lot of small ones and we wonder how fast they grow, etc...
Any info would be gladly received.
Yours in nature,
Des Fuller,
Port
Pirie.”
We sent the following reply to Des:
-
“Hi Des,
It has been
suggested that razorfish are now threatened or endangered. Sorry that we cannot
help you re life cycle, etc.. Suggest that you contact
SARDI at sardi@saugov.sa.gov.au .
Cheers,
Steve”
Des soon
replied as follows: -
“Hi Steve,
Thanks for your
referral to SARDI. Suzanne was very helpful and has sent me some papers on the
findings of surveys done in Gulf St Vincent, and it
has answered all our questions.
Thanks again.
Regards,
Des”
That last enquiry had been the
second recent one regarding razorfish. In September 2004 Adam Browne had sent
us an email request. These details were published in our March 2005 Newsletter
(No.319): -
“Hi,
I hope you don’t
mind general enquiries; I'm looking for information on razorfish, which I'm
told are a kind of shellfish that lives in South Australian waters. I was
wondering if there's anything you could tell me about their distribution around
the Australian coast, especially here in Victoria, and whether they are an
endangered species or are allowed to be fished.
Thank you,
Adam Browne”
We sent the following email reply to
Adam: -
“Dear Adam
You are correct
about razorfish being a kind of shellfish that lives in South Australian
waters. It seems that they occur across southern Australia from WA to NSW,
usually in seagrass beds or sand flats in bays and estuaries. They are not
considered to be an endangered species and they are allowed to be fished in SA.
There are, however, bag limits for the species Pinna bicolor
in SA waters. The department responsible for SA’s fishing regulations, Primary Industries and Resources SA (PIRSA),
currently applies a bag limit of 50 for this species and a boat limit of 150.
Check with your own State’s authorities for fishing regulations in Victoria.
Below are some more details about razorfish: -
Razorfish are
marine bivalve molluscs from the family Pinnidae.
They are also known as Pinna, Razor-shells, Pen Shells and Fan Shells. A simple
description of razorfish can be found in “Seashells of the World” (A Golden
Guide). This little book says that they are “large and fragile, live in soft
sand anchored by a silky byssus”. A byssus is a tuft of dark brown threads*.
A more
complicated description about them is found in “Molluscs” by JE Morton. This
book says that “They have long and wedge-shaped equal valves, and are unique in
being embedded upright in the sand and secured there by a byssus.
Each byssus thread is attached to a sand particle,
and the whole structure gives great stability in a soft substrate. The fan
shell is immobile and the foot and anterior end are greatly reduced. The
posterior or uppermost part of the shell is broad and triangular, composed of
horny conchiolin, only thinly calcified. There is a
wide mantle gape at the broad end, with thickened lips, and,
. . . there are efficient ciliary and mucous
tracts for cleansing the mantle cavity of sediment. The greater part of the
mantle in Pinna is free of attachment to the shell, and its edges can be deeply
withdrawn and protected from injury by special pallial
retractor muscles”.
The book
“Australian Seashores” by Isobel Bennett says that the Pinna are
known as razor-shell because of their sharp (ventral) edges. When these sharp
edges are all that protrudes from sand they can cause serious injury to bare
feet.
*The original
version of “Australian Seashores” by WJ Dakin gives
more information about razorfish and quite some details about the byssus. It refers to the byssus
as “a curious bunch of anchoring threads”. It goes on to say that “The
production of this byssus is a function of the foot
found only in bivalve molluscs. The byssal threads
are secreted by a gland in the foot, which thus becomes an organ for
attachment”.
We hope that this
information helps.
Yours sincerely,
Steve Reynolds
Secretary
Marine Life
Society of SA”
Other request details that have been
published in our Newsletter include the following one received in October 2003
about a Catfish sighting at the Port Noarlunga Reef. The enquiry was sent on to
us by James Brook. It was published in our March 2004 Newsletter (No.308): -
“Hi James
I'm not sure if
this is of interest to you or Reefwatch, but I'll let you know in case it is. On a night dive at Noarlunga Reef last night we saw an unusual fish
(we thought was unusual anyway). At first we I thought it was a rock
ling but it had more than two barbels (and I think
more than four as well). I believe from my fishing book that it was an Estuary
Catfish (Cnidoglanis macrocephalus)
as the body was more rock ling shaped than the more stout bodied eel-tailed
catfish (which is freshwater anyway). I just thought
that this was an unusual sighting as Noarlunga reef is neither
an estuary or brackish water which I believe they are found in. Also,
according to the species location in my book, they are found in Queensland /
Victoria and Western Australia. They're probably very common here but I haven't
seen one before. Also it was about 1-1.2m, so quite big as well. We found it
about 100-200m north of the jetty. If you can give me any feedback on whether
this is an estuary catfish, and how common they are around here, I would
appreciate it. Is there a database that this type of thing goes onto, on the
website, or do I just email you the info? Hope this is of some use.
Cheers
Nick”
James wanted a response from me and
so I sent him the following email reply: -
“Hi James
I keep my own
database of fish sightings at Port Noarlunga reef. I have recorded Estuary
Catfish for the area. These are a marine species and they can be quite large.
They occur in estuarine and coastal waters. They should have 8 barbels on their head. Max size is said to be just 91cm. Everything underwater looks 1/3 bigger i.e. 1.2m.
Steve”
Philip Hall reminded me of various
articles published in our Newsletters and Journals so a later email (to Nick?)
read: -
“Hi Nick,
Further to my
last email re your catfish sighting, David Muirhead has written an article
about a catfish sighting at Port Noarlunga. See the article “A Footbridge Too Fra” in either our December 2000 MLSSA Journal or our March
2003 Newsletter. Both publications can be viewed on our web site at http://www.mlssa.asn.au .
Steve”
Then there was a request for
information about Moonlighters. Details were published in our July 2004
Newsletter (No.312): -
“ We recently
received an email requesting information. It read: -
“Good evening!
I am trying to
identify certain species of fish that may change their sex from either male to
female or otherwise, during their lifetime. Does the Moonlighter come into this
category? I do believe that it comes in the category of SEXFASCIATUM
? Does this mean they are capable of this transaction?
Any information
would be valuable in my studies.
Kind regards,
Leigh Hart,
San Remo, VICTORIA
I sent off the following reply: -
“Hi Leigh
Re. your
enquiry about the Moonlighter changing sex. I have not heard
of this occurring in Moonlighters. I note that you are intrigues over the
scientific name of Vinculum seaxfasciatum. Anearlier name for the same fish was Chaetodon
sexfasciatus. But none of this has anything to do
with changing sex. It’s like the NZ version of six. Sex is actually Latin for
six. In this case it probably refers to the six bands that give the Moonlighter
its other name of Six-banded Coral Fish. We would love to hear of your findings
regarding other fish that change sex.
Regards,
Steve Reynolds
MLSSA”
You may have noticed that we receive
enquiries from SA, interstate and overseas. The following message came from
overseas on 16th May 2005:-
“Dear Sir,
I am
currently studying in the ENITA of Bordeaux in FRANCE (a university level
agricultural institute awarding a five year degree equivalent to a Master of
Science) and I’m looking for a 2-3 month internship in Australia for this
summer 2005 (between early June and early September).
I have done some
research over the internet and with some of my colleagues/teachers in order to
find contacts and information to do this internship. I am quite interested in
your organization, its research domains and the research you are currently
working on.
This internship
is required by my school but the subject is of my own choice and thus I would
like to improve my theoretical and practical knowledge of marine biology
(reefs, animals and plants) or aquaculture (any kind of fisheries: fish, sea
shells, molluscs, etc). I would therefore like to join an existing research
and/or development team in one of those domains in order to try to help in
field studies and results analysis and interpretations.
Please find
attached my Curriculum Vitae (better seen without table gridlines), and
it would be wonderful if you could find a placement for me. I speak fluent
English and I am prepared to take care of travel expenses and living expenses
in Australia if necessary.
Thank you for
your help.
Yours faithfully,
Jérémy Lescot”
We sent the following reply to
Jeremy: -
“Hi Jeremy
The MLSSA
consists of volunteers only. Any one is able to become a member and join in our
activities if that is any help to you.
Regards
Steve Reynolds”
This enquiry came in from overseas
on Wednesday 25th May 2005 (at 5:00AM!).
“My name is Susan
Hieter and I am currently teaching at Kutztown High
School in Kutztown, Pennsylvania for the past 12 years. This summer (end of
July 2005) I will be taking a science teaching at the International School of
Aruba teaching all the sciences from grades 7 to 12. The school was taken over
by the International School Services out of Princeton, New Jersey about 2 years
ago. The school has students from the United States, Canada, the Netherlands,
the United Kingdom, South America, the Caribbean, etc.
I am writing to
you to get any resources and/or ideas that I may take with me to help with the
students in developing the programs of science. Specifically,
marine science, which will be taught to the 11th and 12th grade students.
I am also looking to start a scuba club where we can monitor the reefs and
their environment. I would appreciate any and all materials that you can send
me. Also, the school and myself would be very
interested in becoming members of your organisation. If you have any
suggestions or comments, please email me or send me information. I have
enclosed my new school address (as of July 25, 2005) and my home address (until
July 18, 2005). Thank you so much for
all of your help and effort. I hope to broaden the students
minds with this information.
Sincerely,
Susan B. Hieter
Science Teacher
(7th-12th grade)
International
School of Aruba
Seroe Colorado
San Nicolas
Aruba "Dutch
Caribbean"”
We sent the following reply: -
“Hi Susan (Scuba
Sue)
Scuba Steve here. Great to hear from you. Re becoming
a member of the Marine Life Society of SA, that would be impractical. You
could, however, maintain contact with us by regular reference to our web site
at http://www.mlssa.asn.au . You will
find our monthly newsletters and annual journals posted there. Other pages on
our site cover our annual calendars of SA marine life, our Photographic Index
of SA marine life and our library, plus lots more. We suggest that you contact
Reef Watch at the above address to find out more about reef monitoring. Please
keep in contact and let us know how you get on. Good Luck.
Regards
Steve Reynolds”
Susan (Scuba Sue) soon sent a message
back to Scuba Steve as follows: -
“Hi Scuba Steve,
Thanks for the
info and I just emailed Reef Watch. Thanks again and I'll let you know how
things work out. It might be a while since I probably will be going a little
crazy the first few months.
Scuba Sue”
On 4th June 2005 the
following message was sent to us from overseas: -
“Good morning,
My name is
Alessandra Tomasini and I´m
an Italian biologist graduated to the Universitá degli Studi of Genoa. I´m looking for
a proficiency and interesting opportunity abroad in marine biology.
During university I conduct a field research project on bottlenose dolphin in Genoa´s water for my thesis and now I´d
like to continue on this way in order to gain more experience on research
techniques. I´m available for stage, internship, PhD on population dynamic,
ethological studies or everything else on marine field. Can your
organisation give me this opportunity? What (do) I have to do in order to
obtain this possibility?
Thank you in
advance.
Yours
faithfully.
Alessandra”
We sent the following reply to
Alessandra: -
“Hi Alessandra
Members of the
MLSSA are all volunteers. As a member you would be able to join in our
activities or involve us in your own. We do have dolphins on hand and also
members working with dolphins. You can find out more about our Society at http://www.mlssa.asn.au .
Regards
Steve Reynolds”
It is pleasing that we are able to help people out with
their enquiries regarding marine life topics. It is good to see (hear?) that
there is considerable worldwide interest in marine life by the public.
Further Recent Requests to Our Society for Information
by Philip
Hall
Subject: Fiddler
Ray Photo.
Hello There,
I am writing to
request the use of one of your photos of a Southern Fiddler Ray. The one
on your website that most appeals to me is
1258.jpg. If you have others available that I could preview, I would be
interested in comparing them to 1258.jpg.
I am the Curator
of Aquatic Life at the Pittsburgh Zoo & PPG Aquarium. The Pittsburgh
Zoo & PPG Aquarium is a private non-profit organization dedicated to the
conservation and education of wildlife. We have recently renovated a
stingray exhibit so that it is now a touch tank. In combination with that,
we have incorporated a PowerPoint slide show projected onto the wall behind the
exhibit that shows the different species in the tank. We have a couple of
Fiddler Rays that are soon to be added to the exhibit and so I am looking for a
nice image to include in the slide show.
As it is a
computer projector generating the images on the wall, the image we would be
looking for would only need to be of a medium resolution (i.e. around 1 Meg
file size) as that is the limits of the projectors
resolution.
Please let me
know what would be involved from our part to acquire that image. Thank
you very much and I look forward to hearing from you.
Very truly yours,
Allan.
Allan Marshall
Curator of
Aquatic Life
Pittsburgh Zoo
& PPG Aquarium
My reply read as follows:-
The Committee agreed
to your request and the picture will be scanned and sent to you by another
member.
We do feel that
touch tanks are not the most suitable way to educate the general public and ask
that their use be discontinued as soon as possible. We can give detailed
explanations if needed.
We would
appreciate a note that MLSSA supplied the picture somewhere with perhaps a
reference to our website address and ask for $25 Australian as our usual
copyright fee for non-profit organizations be sent by
electronic transfer. (The supplier of the picture will advise you of the bank
account details.)
Yours sincerely
Philip Hall
(President)
This was the prompt reply from
Allan:-
Hey Philip,
Thank you very
much for the usage of the photo. I completely understand your reservations
regarding touch tanks. Please allow me to try to allay your fears
regarding the animals’ welfare.
I am not sure
what types of “touch tanks” you and the members of your committee have seen
before. There are small versions in public aquariums that usually only
contain a few invertebrates that have no opportunity to escape handling by the
public. I agree that these can be quite stressful to the animals and that
is certainly not the design or intention of our exhibit. The tank shape
and size, life support systems and monitoring by staff and volunteers are
specifically designed to ensure the safety and health of the animals for the
long term.
This particular
tank is about 5 meters by 4 meters in horizontal dimensions and about 80cm
deep. At one end there is a shallow section that is about 40cm
deep. People have access to only two sides of the exhibit. The
design is such that the stingrays are only gently touched if they choose to
swim or sit in the shallow area at one end. If they do not wish to be
touched, they have ample room to swim and/or lay on the bottom
undisturbed. We currently have five species on exhibit and they have all
been doing very well. Some of the rays have been in this exhibit for over five
years while others have been introduced over the past year to increase the
species diversity. Some species are less inclined to be patted and tend
to spend most of their time out of reach of our visitors, while others actually
spend most of their time hanging around in the shallows for the attention.
Of course, we also have an attendant at the exhibit at all times that it is
open to monitor both the public and the animals to be sure that there is no
abusive contact.
Considering that
Pittsburgh is so far from the coast, there many people from the region who have
never seen the ocean and would never have an opportunity to get close to marine
life and make that “connection”. As you can imagine, it is difficult to
encourage conservation of the oceans when our visitors are so far removed from
the issues that face you and so many others that live on or near the shore (I’m
from Brisbane originally and grew up around Moreton Bay and the Sunshine Coast
– yes, I miss it a lot!). The chance to touch a stingray is something
that many of our visitors are enthralled by and they keep that experience with
them for many years. The PPG Aquarium as a whole,
and the stingray exhibit particularly, is a great tool for changing peoples
attitudes and making them aware that their actions do affect more than just
their local surrounds.
Thank you again
for your help with the image. I look forward to receiving it and will
ensure swift payment when the account details are sent to me.
Take care,
Allan.
The next one was a worry and
entailed many email and much phone activity by me. I will include just a few
email items:-
I am writing to
you in the hope you can give me a contact number for someone who could help an
orphaned baby seal at Seal Bay on Kangaroo Island.
My daughter has
just phoned me after visiting Seal Bay, very distressed because they saw a baby
seal there which had lost its mother and the rangers said it was not their role
to intervene. They would simply watch it starve to death which would take
about a week.
She is at the
moment flying back to Adelaide but is desperate to find some way to help this
animal. I am in Brisbane so I have no knowledge of organisations working
at Kangaroo Island.
Surely if the
rangers cannot do anything else, they would ensure the animal died as quickly
and humanely as possible and not watch it starve to death.
I do hope you can
suggest a contact number for me.
Thank you so much
Marjorie Rayment
I sent out a general email to MLSSA
members:-
Hi Folks,
What do you
suggest MLSSA advises in a situation like this?
Philip
Alex Gaut replied:-
I believe that
Parks has a policy of no intervention (but I’m not sure). If we could
check this, then I believe it is MLSSA’s place to reinforce this policy with
the public. I can understand that this would cause distress for many
people, but if Parks start making exceptions then they have to do it every time
and cracks start appearing in the policy.
My own personal
ethics would agree that the cub should be killed quickly, but I believe that
Parks policy should be checked.
If MLSSA as a
whole does not agree with Parks policy then perhaps we need to do something
about that.
Cheers
Alex Gaut
Vice President,
Marine Education Society of Australasia
octopus@chariot.net.au
0418 921 849
After making enquiries with Parks
and Wildlife on KI I then corresponded with Marjorie Rayment and her reply is
below:-
Dear Philip
Thank you for
your reply.
Elizabeth phoned
the RSPCA and was told there was an arrangement for the rangers to contact them
if there was a seal that needed rescue.
A person who I
believe was a chief ranger also phoned Elizabeth and said the rescue facility
that was usually used was now not available because of the incidence of kennel
cough.
The people in the
group were shocked to hear the ranger say they would watch the seal starve to
death and that it would take about a week. We have always believed
rangers had a policy of humane treatment of animals and that if there was no
hope for an animal to survive the rangers would end its suffering as quickly as
possible.
It is not
acceptable to say they do not interfere with the balance of nature. We
all understand those principles, but we interfere with the balance of nature
all the time - including culling the koalas on the island!
We have seen many
documentaries about seal rescues, so we know there are people who rescue
seals. I had gone to the web hoping to find information about a rescue
group covering Kangaroo Island.
Elizabeth and her
party will leave Adelaide tomorrow (Monday) night probably without knowing
whether anything has been done to rescue the seal. We will continue to
contact people to try to follow up what has happened.
Thank you again
for your reply
Marjorie Rayment
Now follows an email from John Hill
MP:-
Dear Mr Hall
Thank you for your email of 7 March
2005 concerning a sealion pup at Seal Bay as reported to you by Ms Marjorie
Rayment.
The pup in question continues to be
monitored in relation to its health and well being and, at last report, the
animal was observed to be alert, active and feeding.
Seal Bay is home to a colony of wild
Australian Sealions. As such, these
animals are exposed to the hazards and risks that face all wild animals.
Sealion mothers leave their pups on
the beach and undertake foraging trips up to 60km out to sea for approximately
72 hours duration. Pups are left to
their own devices during their mother’s absence and quite often interact with
other animals in the colony including some limited surrogate feeding
activity. At times mothers may be absent
for extended periods well beyond the typical 72 hours, with some recorded
absences of over two weeks. Pups may
struggle during these absences, but successful rearing has occurred following
the mother’s return when pups suckle consistently for the three to four days
the mother is ashore. Consequently,
determining whether an animal has been “orphaned” or not is very difficult. The mother rearing and educating the pup
achieves the best chance of pup survival and successful interaction and
breeding in the colony.
Despite this difficulty, all
necessary practices and procedures are followed in relation to obligations
under the National Parks and Wildlife Act 1972 and the Prevention of
Cruelty to Animals Act 1985. Animals
that have been in serious difficulty in the past, have
been humanely destroyed, however, there are also numerous examples of sealions
fully recovering following a serious mishap.
While I am disappointed that Ms Mayne’s experience at Seal Bay was not as positive as she
had hoped, I am satisfied that the concerns raised by Ms Mayne,
Ms Rayment and you are being appropriately addressed by Department for
Environment and Heritage officers.
Thank you for your interest in this
matter.
Yours sincerely
JOHN HILL
Following
a talk on local marine life Margaret and I gave to the Yankalilla Probus Club I
was asked if I could identify an item found on the local seashore. A few days
later the following picture was emailed to me.
The object was described as having a
waxy feel and I thought it might be Ambergris which is found inside whales.
These animals pass nearby quite frequently. I contacted a firm in New Zealand
which buys and sells ambergris and they were very doubtful.
The next step will be to see if the finder will be
willing for me to take it to the South Australian Museum to one of the experts
there. An ongoing quest at the time of writing.
The unidentified object