Marine life Society
of South Australia Inc
2006 Journal
Number 16
December 2006
THE MARINE LIFE SOCIETY OF SOUTH AUSTRALIA Inc.
Are
you interested in any aspect of marine life? Do you want to learn more about the
underwater world? Are you concerned about pollution of our oceans and
destruction of reefs and seagrass beds? If so MLSSA is for you.
Our
motto is “--- understanding, enjoying and caring for our oceans ---”. These few
words summarise our aims. Members seek to understand our ocean, derive
enjoyment from observations of marine life and are committed to protection of
the marine environment.
Become
a Society member and enjoy contact with others with similar interests. Our
members include divers, marine aquarists and naturalists.
Our
activities include:-
-Studying
our local marine environment
-Community Education
-Underwater
photography
Established
in 1976, MLSSA holds monthly meetings and occasional field trips. We produce
various informative and educational publications including a monthly
Newsletter, an Annual Journal and a beautifully illustrated Calendar showing
only South Australian marine life. Our library is a source of helpful
information for marine enthusiasts.
Through
our affiliation with other organisations (eg Conservation Council of SA
and the Scuba Divers Federation of SA)
we are kept up to date with relevant issues of interest. MLSSA also has close
ties with appropriate Government organisations, e.g. various museums,
universities and libraries.
Everyone
is welcome to attend our General Meetings which are held on the third Wednesday
of every month (except December) at the Conservation Centre, 120 Wakefield
Street, Adelaide. We begin with a guest speaker. After a short break there is
the general business meeting and this may be followed by a slide show if time
permits. The atmosphere is friendly and informal.
We
welcome new members. We have subscription levels for students, individuals,
families and organisations. We invite you to complete the membership
subscription form on our website at:-
http://www.mlssa.asn.au
Or
you may wish to write to the Society for a form, or to complete the one inside
the rear cover of this Journal (or a photocopy) and send it with your payment
to MLSSA.
The postal
address of the Society is:-
MLSSA
Inc.
120
Wakefield Street,
ADELAIDE 5000.
OUR LOGO
The
MLSSA logo features a Leafy Seadragon which is unique to southern Australian
waters. The Leafy is South Australia’s first totally protected fish and is the
State marine emblem. Its beauty surpasses that of any creature found in
tropical waters and, once seen by divers, is amongst the most remembered of
their diving experiences.
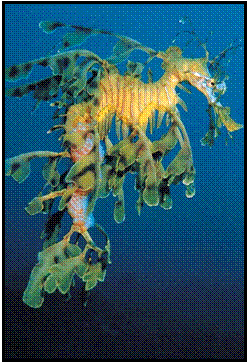
Photograph
courtesy of MLSSA member David Muirhead.
CONTENTS
Three fouling bryozoans species in Adelaide Waters Brian J. Brock
The Marine Life at Port Noarlunga Reef Steve Reynolds
Sepia apama, the giant Australian cuttlefish,
in Whyalla, S.A.
Evan John
Fossil Cave/Green Waterhole Cave (5l81)
Bone Retrieval Dives
Peter Horne
What You Should Know About Great White Sharks Phil Kemp
The Western Blue Groper
Scoresby Shepherd
The Flora and Fauna of Piccaninnie Ponds
and Ewens Ponds
Steve Reynolds
Patagonian Tooth-fish – why all the fuss? Evan John
Save Ewens Ponds!
Gerard Carmody
EDITORIAL
Welcome
to the 2006 edition of the MLSSA Journal. As usual, this replaces the December
monthly Newsletter.
This
edition of the Journal is the largest we have produced and my thanks go the
authors who so willingly gave of their time to create such a wide diversity of
interesting and informative articles.
Members,
ex-members and people only remotely connected with MLSSA have made this edition
possible.
A
wide variety of topics are covered and you should find something here to
interest and intrigue you.
Good
reading and a safe and happy Christmas and New Year to you all.
DISCLAIMER
The
opinions expressed by authors of material published in this Journal are not
necessarily those of the Society.
EDITING: Philip
Hall
PRINTING: Phill
McPeake
CONTRIBUTORS: Brian Brock
Steve
Reynolds
Evan
John
Peter Horne/Dave Albano
Phil
Kemp
Scoresby
Shepherd
Gerard
Carmody
PHOTOGRAPHY: Philip Hall
Ron
Hardman
David
Muirhead
Neville
Skinner
Peter
Horne
Mike
Hammer
Paul
Macdonald
Gerard
Carmody
Kath
Moores
Rudie
Kuiter
by Brian J Brock
Bryozoans
are colonial marine or freshwater organisms. The colony is made up of a few or
many thousands of individuals, each in a little box, cup-like, or tube-like
chamber, which may be more or less calcified. When feeding, a ring of tentacles
is protruded from the protective chamber. Cilia along the tentacles beat in
such a way that food organisms or particles are swept towards the base of the
tentacle bell. When the mouth opens and the pharynx dilates, the food is forced
into the top end of the U-shaped gut by water pressure. Some colonies look like
plants, others are heavily calcified and might be mistaken for coral. Others
form delicate incrustations or branching sculptures on brown or red algae or
marine flowering plants. Living bryozoans are common on most submerged
surfaces; look for them on boat bottoms, pontoons, buoys, jetty piles, rocks,
mangrove pneumatophores and waterlogged branches, hulks, etc. Fossil species
are common in limestone cliffs along the Murray River or around our coasts and
in Mount Gambier limestone.
If living
colonies are put in fresh seawater (for marine species) the feeding currents
and tentacle bells might be seen. A hand lens will help. Following a sea snake
to see living bryozoans on its tail is not recommended.
From mid
1975 until February 1977, I carried out settlement experiments at Outer Harbour
and Angas Inlet. The latter site is warmed by effluent from the Torrens Island
Power Station. My settlement tiles for the longest term experiment, were dark
grey cement aggregate window-sill tiles suspended horizontally, 50cms below
water, beneath pontoon platforms. Pairs of tiles were immersed for a month at
each site, new tiles being put in every fortnight, Tiles were raised after a
month, preserved in 10% formalin seawater, and ancestrulae or young colonies of
each fouling bryozoan were counted under a microscope.
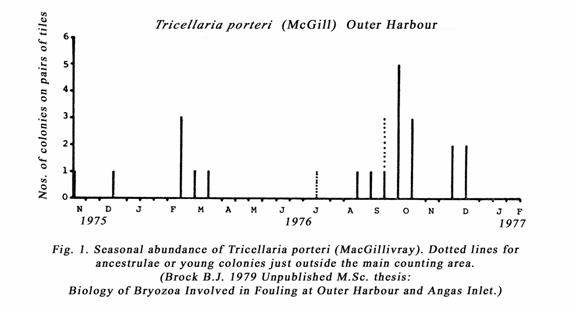
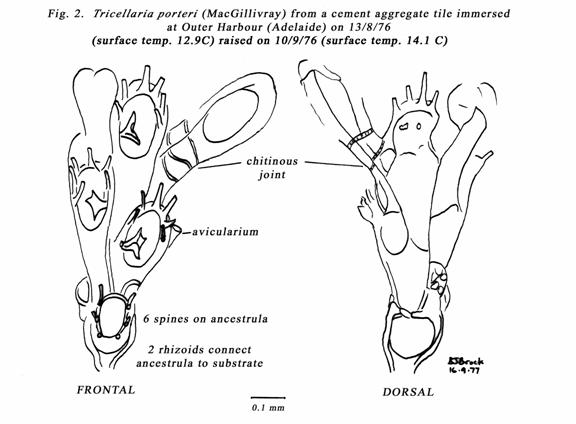
Tricellaria only
occurred at Outer Harbour, the colder water site. Its seasonal abundance is
shown in the histogram (fig. 1). Figure 2, shows a 6-spined ancestrula of the Tricellaria
species and some other details of the young colony. Old colonies were not found
despite regular sampling of pontoon foulers. My settlement tiles could have
been seeded by propagule from some of the fouled yachts or other harbour
installations or mooring facilities. Vessels moored for a long time became
heavily fouled with bryozoans and other benthic invertebrates and algae etc.
On June 7th
1889, a paper by P. H. MacGillivray titled “On some South Australian Polyzoa”,
was read to the Royal Society of South Australia.
Menifera
Porteri, was one of four new species described and illustrated
(Plate11, figs. 1-1b). This is the species later known as Tricellaria
porteri (MacGillivray). MacGillivray described and illustrated the ooecia
as “large, rounded, with a row of foraminera along the upper edge”. The
ancestrula is not described, but I believe this is the species that settled on
my Outer Harbour tiles in 1976. Specimens MacGillivray saw, grew on algae. I
have not seen ovicellate specimens.
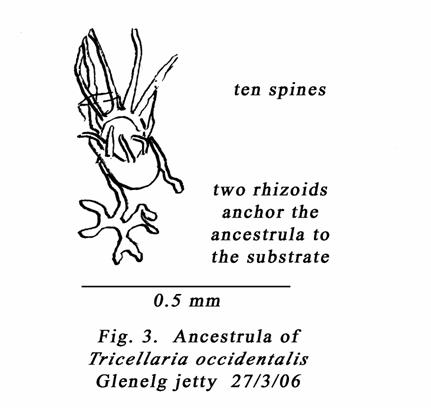
Contrast
the 6-spine count for the ancestrula of T. porteri (MacGillivray), with
the 10-spine count for an ancestrula collected from a Tricellaria band
just above Low Water Spring Tide level on a Glenelg jetty pile on 27/3/06. This
appears to be an ancestrula of Tricellaria occidentalis. See my Fig. 3.
It accords with Mawatari (1951, figs 1A & fig. 7 for T. occidentalis &
Anna Occhipinti Ambrogi & J.L. d’Hondt’s (1994) fig. 2 p141 for Tricellaria
inopinata. The 1994 paper deals with the invasion of Venice Lagoon by a Tricellaria
species. Gordon & Mawatari (1992) considered T. inopinata and Menipea
Porteri MacGillivray, 1889, to fall within the range of variations for T.
occidentalis.
Anna Occhipinti Ambrogi (1991) says
of her T. inopinata in Venice Lagoon, Tricellaria “was never
found deeper than the Low Water Spring Tide”. This applies for my T.
occidentalis on Glenelg jetty piles. If I could find colonies of Tricellaria
conforming to MacGillivray’s description, below Low Water Spring Tide level on
Glenelg jetty piles, it would be fairly convincing proof that we do have two
different species of Tricellaria in Adelaide waters. Ancestrulae &
ovicellate colonies would be helpful. A settlement tile I fastened to a pile on
27/2/06 disappeared. It was KESAB week.
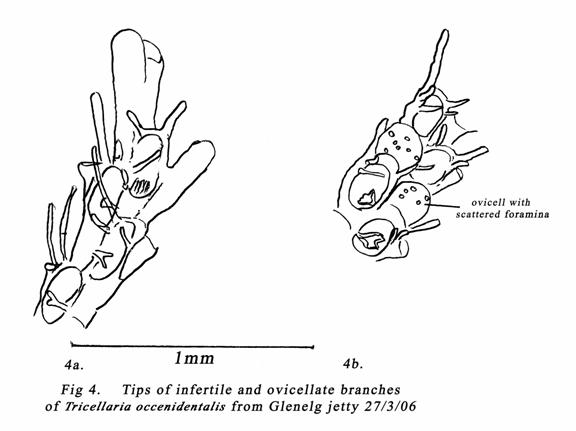
Fig
9.15(c) of Bock (1982) shows ovicells of Tricellaria porteri with
several scattered foramina. Such an arrangement is more characteristic of occidentalis
as shown in Mawatari (1951) figs 1E & 1H, Nielsen (1985) fig 3, &
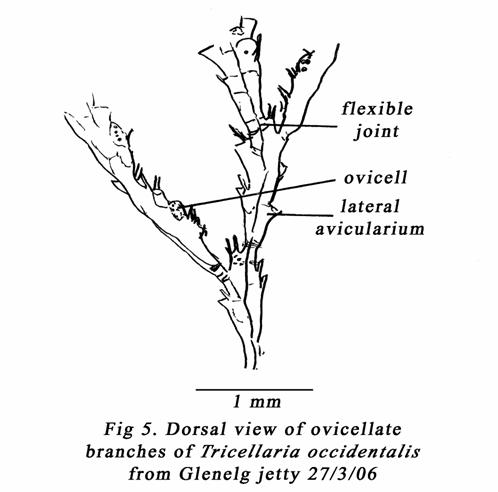
Gordon & Mawatari 1992 plate 6F. See also my fig 4b. Flexible joints at the
base of the branches are shown in my fig 5. Brock (1985) has illustrated
several South Australian fouling bryozoans, including T. porteri (MacGillivray,
1889) but ovicells & ancestrulae of Tricellaria were not shown.
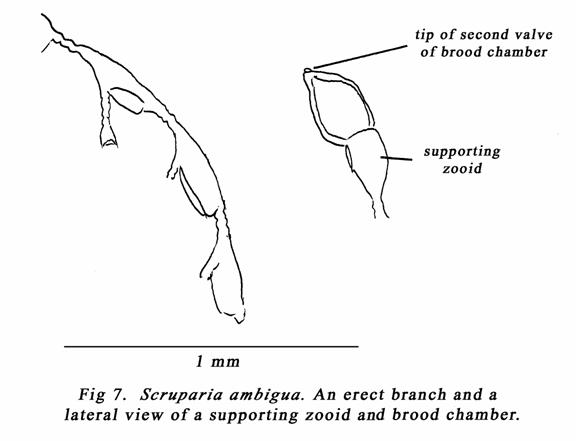
The third fouling bryozoan found
recently, was Scruparia ambigua (d’Orbigny). The specimens were growing
on drift red alga (Ceramium) from Osborne Beach south of North Haven
marina on 6/8/06. A single line of adherent zooids buds off erect monoserial
branches of zooids some of which might terminate in brood chambers. See my
figs. 6 & 7, Ryland (1965) pp22 & 23, & Ryland & Hayward (1977)
pp 50 & 51. The brood chambers have two valves and look a bit like a
Moroccan gate archway, or bishop’s mitre. The line where the valves join each
other can be seen in the frontal view of the supporting zooid (my fig. 6).
Scruparia
ambigua and Scruparia chelata (Linnaeus) used to be
confused, but in the latter species, the erect branches bud from swollen parts
of an adherent stolon (Ryland & Hayward, 1977 pp 52 & 53). Scruparia
species are common enough as foulers of ships and on settlement plates, but
because of their weak habit, are not particularly troublesome. They are often
found on other more robust foulers (Gordon & Mawatari, 1992, p17).

REFERENCES
Bock P.E.
(1982) Bryozoans. Chapter 9 of S.A. Shepherd & I.M. Thomas (Eds.) Marine
Invertebrates of Southern Australia Part I (S.A. Govt. Printer).
Brock
B.J. (1985) South Australian fouling Bryozoans. In: C. Nielsen & G.P.
Larwood (Eds.) Bryozoa: Ordovician to Recent pp 45-49. (Olsen & Olsen).
Gordon D.P. & Mawatari S.F.
(1992) Atlas of Marine Fouling Bryozoa of New Zealand Ports & Harbours.
(Miscellaneous Publications of the New Zealand Oceanographic Institute No.
107.)
MacGillivray
P.H. (1889) On some South Australian Polyzoa. Transactions & Proceeding
& Report of the Royal Society of South Australia vol.12 for 1888-89 pp
24-30 & Plate II.
Mawatari
S. (1951) On Tricellaria occidentalis (Trask), one of the fouling
Bryozoans of Japan. (Miscellaneous Reports of the Research Institute for
Natural Resources Tokyo No. 22 pp 9-16).
Nielsen
C. (1985) Ovicell formation in Tegella & four cellularioids
(Bryozoa, Cheilostomata). In: C. Nielsen & G.P. Larwood (Eds.) Bryozoa:
Ordovician to Recent (Olsen & Olsen) pp 213-220.
Occhipinti
Ambrogi (1991) The spread of Tricellaria inopinata into the Lagoon of
Venice: an ecological hypothesis. In: Bigey F.P. (Ed.) Bryozaires Actuels et
Fossiles: Bryozoa Living & Fossil (Bull. Soc. Sci. Nat. Ouest Fr., Mem.
H.S.1) pp. 299-308.
Occhipinti
Ambrogi & J-L d’Hondt (1994) The invasion ecology of Tricellaria
inopinata into the Lagoon of Venice: morphological notes on the larva &
ancestrula. In P.J. Hayward, J.S. Ryland & P.D. Taylor (Eds.) Biology &
Palaeobiology of Bryozoans (Olsen & Olsen) pp139-144.
Ryland
J.S. (1965) Catalogue of Main Marine Fouling Organisms vol. 2 Polyzoa.
(O.E.C.D. Paris).
Ryland J.S. & Hayward P.J.
(1977) British Anascan Bryozoans (Academic Press).
The Marine Life at Port Noarlunga Reef
by Steve Reynolds
The
History of the Port Noarlunga Reef Aquatic Reserve
The Port
Noarlunga reef (and Onkaparinga estuary) was named as one of six Aquatic
Reserves established in South Australia on 30th November 1971. The
six reserves were declared under the SA Fisheries Act 1971. The Barker Inlet
was declared as SA’s seventh Aquatic Reserve in 1973. The seven aquatic
reserves were created in order to retain the natural animal and plant
communities on some reefs, and in some estuarine mangroves and seagrass areas.
More Aquatic Reserves were declared in December 1980 and others followed later
on.
The
reasons given for establishing marine aquatic reserves were to: -
Have
sanctuaries where aquatic fauna and flora may flourish with minimal human
interference and predation.
Provide
recreational areas where natural marine communities can be readily examined,
appreciated and photographed.
Maintain
marine communities in an untouched state so that they can be scientifically
examined and studied over extended periods.
Establish breeding reservoirs for
certain species of reef fish for repopulating areas outside the reserves where
spearfishing takes place.
The Port
Noarlunga reef had been protected against spearfishing since 1965.
My Own
Diving Experience at The Reef
I did my
very first scuba dive at Port Noarlunga reef to complete my basic scuba
certificate on 4th February 1978. It was more a dive under the
jetty. My instructor and I merely swam to the reef and back along the jetty
once or twice. After that, most of my dives were made with fellow members of
the Marine Aquarium Research Institute of Australia (SA Branch), or MARIA SA.
Since the main purpose of our diving back then was to collect specimens for our
temperate marine aquariums, I wasn’t very interested in diving at Port
Noarlunga reef because it was an Aquatic Reserve. I did, however, manage to do
a dive there at the end of that same year. I dived with fellow MARIA member,
Gavin Roberts. My dive logbook does not show any other details and I don’t have
any recollection of the dive at all.
I
continued to do most of my diving with fellow MARIA members at sites where we
could collect aquarium specimens. We did manage to dive at the Aldinga dropoff,
however, in 1981. It was almost three years before I returned to the reef for
another dive.
In
October 1981 nine MARIA members conducted a survey dive at the Port Noarlunga
reef. Phill McPeake, as our Diving Officer at the time, wrote a brief report
about the dive in our November 1981 Newsletter (No.54). Our two Research Officers
at the time were the organizers of the survey. They issued each of the nine
divers with an underwater slate, split them into four teams and gave each team
a designated area for them to record all of their marine life sightings.
According
to my dive logbook, we enjoyed excellent conditions for the day. The weather
was sunny, the tide was low and the sea was calm. I estimated the visibility to
be about 20’ (6m).
I was
teamed up with the DO for the dive. We were instructed to swim north along the
inside of the reef and then to return to the jetty on the outside of the reef.
I enjoyed our dive which was only marred by the loss of the DO’s underwater
slate. We could only jog his memory about some of the creatures that he had
seen.
Back at
the jetty we rejoined the rest of the divers who had returned from their
designated areas. All of the slates were handed over to the Research Officers.
They later transferred the details into our club dive logbook. To the best of
my knowledge, the details have not been published in any form to date.
I did a
night dive with several MARIA members at Port Noarlunga early in 1982. As our
Publications Officer at the time, I wrote a report about this dive for our
March 1982 Newsletter (No.58).
I didn’t
return to Port Noarlunga until early 1985 when I did another night dive with
fellow (now MLSSA) members. This dive was reported in our February 1985
Newsletter (No.92). Our dive started after 10.30pm and lasted until well after
midnight. It was almost two years before I returned to Port Noarlunga reef
again. I had done some 79 other dives during those two years. Clearly, Port
Noarlunga reef was not high on my list of preferred dive sites at this time. I
had managed only six dives there in nine years. And that’s the way that my diving
continued, just an occasional dive at Port Noarlunga reef. I can’t take all of
the responsibility for that, however, since most dives were with a buddy.
In the
1990s I managed to go almost six years without a dive at Port Noarlunga. Then
everything changed all of a sudden. At the end of 1997 I gained a new dive
buddy and since then we have often dived together at Port Noarlunga. Despite
not diving regularly, I have managed to do some 30 dives there since then (not
all with the same buddy). I now realize that I would have missed out on a lot
by not diving there much in the earlier years.
And what
are my best memories of dives at Port Noarlunga? I love seeing Rainbow Fish,
Blue Devils, large stony corals (Plesiastrea versipora) and Black Cowry
(Cypraea friendii thersites) there. I have seen several species
of nudibranchs and the opisthobranch Bat-wing Sea Slug, Sagaminopteron
ornatum. I have seen Western Cleaner Clingfish, fish cleaning stations, a
sea mouse, Port Jackson sharks and stingrays.
The most
memorable dive at present is the most recent one. In November 2005 we saw a
congregation of about 20 large Port Jackson sharks out from the outer-side of
the reef. We dived on the outside first, then we swam through the gap to the
inside. We saw some of my favourite creatures including Castlenau’s Wrasse,
large Senator Fish, a large spider crab, sea pen and two large Ceratosoma
brevicaudatum nudibranchs. I also managed to swim through two
swim-throughs. All very exciting!
But the
most exhilarating moment for me was when I first jumped off of the reef to dive
the outside. A ‘shadow’ rushed towards me and my heart skipped a beat but I
soon realized that a large seal was swimming all around me. We are often
surrounded by schools of fish under the jetty close to the reef.
In an
article titled “Fish Recorded at Port Noarlunga Reef” which was published in
our December 1987 Newsletter (No.125) I suggested that “The biggest danger from
fish may be whilst ‘handfeeding’ large Leatherjackets”. My dive buddy on 4th
December 1999 had obviously not read my article because he proceeded to feed
cockles to the fish under the jetty and was bitten on the forehead by
leatherjackets. The 21°C water numbed the bites a little at the time but when
he exited from the water his family wondered why he was bleeding from the
forehead.
Society
members did transect dives, led by Kevin Smith, at the reef in October 2004.
During a reconnaissance dive before the transect dives, Kevin found a Sawtooth
Pipefish, Maroubra perserrata. I have only ever seen two before.

Flabellina species of nudibranch sighted at Port Noarlunga reef in 2006
(Paul Macdonald)
Reports
About Port Noarlunga’s Marine Life
Whilst
working for the SA Department of Agriculture and Fisheries in 1975, Dr Hank Duyverman
wrote a paper titled “Ecological Surveys of the South Australian Aquatic
Reserves”. One small part of this report was titled “A Brief Ecological Survey
of the Port Noarlunga Reef”. (Much of Duyverman’s work at Port Noarlunga was
done at the southern end of the reef.)
In 1987 I
wrote the article titled “Fish Recorded at Port Noarlunga Reef” which was
published in our December 1987 Newsletter (No.125). In that article I wrote
that “More than 60 species of fish have been sighted around Port Noarlunga reef
and most of these are fairly common. The commonest species seen here are sweep,
bullseyes, coralfish, leatherjackets, drummer, Dusky Morwong, Magpie Perch, Red
Mullet, Sand Mullet, Scaly Fin, trachinops, cardinalfish, and weedfish. Most of
these fish are resident reef dwellers and they make excellent photographic
subjects for divers and snorkellers with underwater cameras. Many species of
invertebrates such as sponges, anemones, shells, crustaceans and starfish are
also common around the reef which is one of the natural wonders of our
coastline”.
The
article included a list of 64 species of fish that have been recorded at the
reef by our members. Much of these details came both from Hank Duyverman’s
report “A Brief Ecological Survey of the Port Noarlunga Reef” and Denise
Warren’s article “The Fish of Noarlunga Reef” in our MARIA Journal (No.1),
October 1979.
Both of
these are included in our library. “A Brief Ecological Survey of the Port
Noarlunga Reef” is item mlssa 2002. MARIA Journal No.1, which includes the
article “The Fish of Noarlunga Reef”, is in our Journal folder.
Both
included fish and plant and invertebrate lists. We can now add both Western
Cleaner Clingfish and Sawtooth Pipefish to the list of fish species recorded
there.
The
plants and invertebrates list from Duyverman’s report was reproduced in
Warren’s article, although it was said to be “a more extensive list of plants
and creatures”. The only obvious additions to the list were the murex shell Pterynotus
triformis, the helmet shell Cassis fimbriata and “numerous scallops
and brachiopods”.
Both
lists had some errors and some errors occurred during the re-writing for
Warren’s article. Many of the details in the lists are now out of date due to
scientific changes.
Our
second Journal, MARIA Journal Vol.1, No.2, November 1979 included some comments
on the list by one of our Research Officers, Evan John. He felt that common
names should be listed first if they are to be of “value to the average person
interested in marine biology”. He also discussed several other points. Some
errors occurred in the publication of these comments. Some errors have also
occurred in reference books that I have used.
In this
article I have attempted to update both lists (now three lists), without making
any errors, taking Evan John’s comments into account.
Many
thanks go to Hank Duyverman, Bob Baldock, Kevin Smith and Paul Macdonald for
their assistance with this article.
Here then are my lists: -
|
LIST OF FISH SIGHTINGS AT PORT
NOARLUNGA REEF |
|||
|
Family |
Genus |
Species |
Common
Name |
|
Antennariidae |
Rhycherus |
filamentosus |
Tasselled
Anglerfish |
|
Aploactinidae |
Aploactisoma |
milesii |
Velvetfish |
|
Apogonidae |
Vincentia |
conspersa |
Southern
Cardinal Fish |
|
Arripididae |
Arripis |
trutta |
Eastern
Australian Salmon |
|
Blenniidae |
Parablennius |
tasmanianus |
Tasmanian
Blenny |
|
Bovichtidae |
Bovichtus |
angustifrons |
Thornfish |
|
Callionymidae |
Foetorepus |
calauropomus |
Common
Stinkfish |
|
Carangidae |
|
|
Trevally
Sp. |
|
Chaetodontidae |
Chelmonops |
curiosus |
Western
Talma |
|
Cheilodactylidae |
Cheilodactylus |
nigripes |
Magpie
Perch |
|
Cheilodactylidae |
Dactylophora |
nigricans |
Dusky
Morwong |
|
Clinidae |
|
|
Weedfish
Spp. (4 ) |
|
Congridae |
Conger |
wilsoni |
Eastern Conger
Eel |
|
Dasyatididae |
Dasyatis |
brevicaudata |
Smooth
Stingray |
|
Dinolestidae |
Dinolestes |
lewini |
Longfin
Pike |
|
Diodontidae |
Diodon |
nichthemerus |
Globe
Fish |
|
Enoplosidae |
Enoplosus |
armatus |
Old Wife |
|
Girellidae |
Girella |
tricuspidata |
Blackfish/Luderick |
|
Girellidae |
Girella |
zebra |
Zebra
Fish |
|
Gobiesocidae |
Aspasmogaster |
tasmaniensis |
Tasmanian
Clingfish |
|
Gobiesocidae |
Cochleoceps |
bicolor |
Western
Cleaner Clingfish |
|
Gobiidae |
|
|
Goby Spp. |
|
Hemiramphidae |
Hyporhamphus |
melanochir |
Garfish |
|
Heterodontidae |
Heterodontus |
portusjacksoni |
Port
Jackson Shark |
|
Kyphosidae |
Kyphosus |
sydneyanus |
Silver
Drummer |
|
Labridae |
Achoerodus |
gouldii |
Western
Blue Groper Wrasse |
|
Labridae |
Austrolabrus |
maculatus |
Black-spotted
Wrasse |
|
Labridae |
Eupetrichthys |
gloveri |
Slender
Wrasse |
|
Labridae |
Pictilabrus |
laticlavius |
Senator
Wrasse |
|
Labridae |
Pseudolabrus |
parilus |
Brown-spotted
Wrasse |
|
Microcanthidae |
Tilodon |
sexfasciatum |
Moonlighter |
|
Monacanthidae |
Acanthaluteres |
brownii |
Spiny-tailed
Leatherjacket |
|
Monacanthidae |
Acanthaluteres |
vittiger |
Toothbrush
Leatherjacket |
|
Monacanthidae |
Brachaluteres |
jacksonianus |
Pygmy
Leatherjacket |
|
Monacanthidae |
Eubalichthys |
mosaicus |
Mosaic
Leatherjacket |
|
Monacanthidae |
Meuschenia |
hippocrepis |
Horseshoe
Leatherjacket |
|
Monacanthidae |
Parika |
scaber |
Velvet
Leatherjacket |
|
Mugilidae |
Myxus |
elongatus |
Sand
Mullet |
|
Mullidae |
Upeneichthys |
vlamingii |
Southern
Goatfish |
|
Odacidae |
Odax |
acroptilus |
Rainbow
Cale |
|
Odacidae |
Odax |
cyanomelas |
Herring
Cale |
|
Odacidae |
|
|
Weed-Whiting
Spp. |
|
Orectolobidae |
Orectolobus
Sp. |
|
Wobbegong
Shark Sp. |
|
Ostraciidae |
Aracana |
ornata |
Ornate
Cowfish |
|
Pegasidae |
Pegasus |
lancifer |
Sculptured
Seamoth |
|
Pempheridae |
Pempheris |
klunzingeri |
Rough
Bullseye |
|
Pempheridae |
Pempheris |
multiradiata |
Common
Bullseye |
|
Pentacerotidae |
Pentaceropsis |
recurvirostris |
Long-snouted
Boarfish |
|
Platycephalidae |
|
|
Flathead
Spp. |
|
Plesiopidae |
Paraplesiops |
meleagris |
Western
Blue Devil |
|
Plesiopidae |
Trachinops |
noarlungae |
Trachinops |
|
Plotosidae |
Cnidoglanis |
macrocephalus |
Estuary Catfish |
|
Pomacentridae |
Parma |
victoriae |
Scaly Fin |
|
Scorpaenidae |
Glyptauchen |
panduratus |
Goblinfish |
|
Scorpidae |
Scorpis |
aequipinnis |
Sea Sweep |
|
Scorpidae |
Scorpis |
georgianus |
Banded
Sweep |
|
Serranidae |
Othos |
dentex |
Harlequin
Fish |
|
Sillaginidae |
Sillaginodes |
punctatus |
King
George Whiting |
|
Sparidae |
Chrysophrys |
auratus |
Snapper |
|
Syngnathidae |
Solegnathus |
spinosissimus |
Spiny
Pipehorse |
|
Syngnathidae |
Stigmatopora |
argus |
Spotted
Pipefish |
|
Syngnathidae |
|
|
Seahorse
Sp. |
|
Syngnathidae |
Maroubra |
perserrata |
Sawtooth
Pipefish |
|
Tetraodontidae |
Tectractenos |
glaber |
Smooth
Toadfish |
|
Tetraodontidae |
Torquigener |
pleurogramma |
Banded
Toadfish |
|
Trachichthyidae |
Trachichthys |
australis |
Roughy |
|
? |
|
|
Flounder
Sp. |
LIST OF
INVERTEBRATE SIGHTINGS AT PORT NOARLUNGA REEF (Part chart only)
|
Family |
Genus |
Species |
Description |
|
|
Pennaria |
wilsoni |
Hydroids |
|
|
Silicularia |
campanularia |
|
|
Sertulariidae |
Sertularia |
Spp. |
|
|
Plumulariidae |
?Aglaophenia |
Sp. |
|
|
|
?Aequorea |
Sp. |
|
|
|
3 other
unidentified |
species |
|
|
|
Aurelia |
aurita |
|
|
|
1 variety
of blue |
bottle |
|
|
|
Oulactis |
muscosa |
Speckled
or Shellgrit Anemone |
|
|
Actinia |
tenebrosa |
Waratah
anemone |
|
|
Aulactinia |
veratra |
Green
Anemone |
|
|
Anthothoe |
albocincta |
Anemone |
|
|
Zoanthus |
robustus |
Zoanthid |
|
|
Parerythropodium |
membranaceum |
|
|
|
|
|
Soft
Coral |
|
|
Plesiastrea |
versipora |
Stony
coral |
|
|
?Sarcoptilus |
Sp. |
Sea Pen |
|
|
?Sycon |
Sp. |
Sponge |
|
|
Carteriospongia |
caliciformis? |
Sponge |
|
|
Tethya |
Spp. |
Golfball
sponge |
|
|
many
other unidentified |
species
of |
Sponge -
all colours |
|
|
Triphyllozoon |
monilferum |
Bryozoan |
|
|
Membranipora |
membranacea |
Bryozoan |
|
Ascidiidae |
Ascidia |
sydniensis |
Solitary
ascidian |
|
Ascidiidae |
Herdmania |
momus |
Solitary
ascidian |
|
Ascidiidae |
Phallusia |
depressiuscula |
Ascidian |
|
Clavelinidae |
Clavelina |
cylindrica |
Colonial
ascidian |
|
Clavelinidae |
Clavelina |
moluccensis |
Colonial
ascidian |
|
Pseudodistomidae |
Pseudodistoma |
gracile |
Ascidian |
|
Styelidae |
Cnemidocarpa |
etheridgii |
Ascidian |
|
Styelidae |
Polycarpa |
pedunculata |
Ascidian |
|
Polycitoridae |
Polycitor |
giganteus |
Ascidian |
|
Pyuridae |
Pyura |
australis |
Ascidian |
|
Pyuridae |
Pyura |
irregularis |
Ascidian |
|
Pyuridae |
Pyura |
pachydermata |
Ascidian |
|
Ritterellidae |
Ritterella |
pedunculata |
Ascidian |
|
Holozoidae |
Sycozoa |
cerebriformis |
Ascidian |
|
Holozoidae |
Distaplia |
viridis |
Ascidian |
|
Didemnidae |
Leptoclinides |
imperfectus |
Ascidian |
|
Didemnidae |
Leptoclinides |
maculatus |
Ascidian |
|
|
several
unconfirmed |
or
unidentified |
species,
incl. |
|
Styelidae |
Botrylloides |
leachi |
Compound
Ascidian |
|
Polycitoridae |
Eudistoma |
aureum |
Ascidian |
|
Didemnidae |
Leptoclinides |
rufus |
Ascidian |
|
Pyuridae |
Microcosmus |
stoloniferus |
Ascidian |
|
Didemnidae |
Polysyncraton |
orbiculum |
Ascidian |
|
Agnesiidae |
Rhodosoma |
turcicum |
Ascidian |
|
Styelidae |
Stolonica |
australis |
Ascidian |
|
Polyclinidae |
Synoicum |
sacculum |
Ascidian |
|
Ischnochitonidae? |
Ischnochiton? |
australis |
Chiton |
|
Plaxiphoridae |
Poneroplax |
Sp. |
Chiton |
|
|
8 other
unidentified |
species |
Chiton |
|
Haliotidae |
Haliotis |
rubra |
Black-lip
abalone |
|
Haliotidae |
Haliotis |
roei |
Abalone |
|
Patellidae |
Cellana |
Spp. |
Limpets |
|
|
Montfortula |
Sp. |
|
|
Trochidae |
?Bankivia |
Sp. |
Kelp
shell |
|
Mitridae |
?Mitra |
Sp. |
Mitre
shell |
|
Muricidae |
Pterynotus |
triformis |
Murex |
|
Muricidae |
Dicathais |
orbita |
Cartrut
shell |
|
Cassidae |
Cassis |
fimbriata |
Helmet
shell |
|
Turbinidae |
Turbo |
undulatus |
Common
Warrener |
|
Turbinidae |
Phasianella |
australis |
Pheasant
shell |
|
Fasciolaridae |
Pleuroploca |
australasia |
Tulip
shell |
|
Cypraeidae |
Cypraea |
friendii
(thersites) |
Black
Cowry |
|
|
&
many other unidentified |
species |
Gastropod
shells |
|
Gastropteridae |
Sagaminopteron |
ornatum |
Bat-wing
Slug |
|
Chromodorididae |
Ceratosoma |
brevicaudatum |
Short-tailed
Ceratosoma |
|
Flabellinidae |
Flabellina |
Sp. |
Flabellina
nudibranch |
|
Mytilidae |
Brachiodontes |
rostratus |
Mussel |
|
Mytilidae |
Modiolus |
Sp. |
Mussel |
|
Mytilidae |
Xenostrobus |
pulex |
Mussel |
|
Pteriidae |
Malleus |
meridianus |
Southern
Hammer Mussel |
|
Pectinidae |
Numerous
scallop |
species |
|
|
Loliginidae |
Sepioteuthis |
australis |
Squid |
|
Sepiidae |
Sepia |
apama |
Giant
cuttle |
|
|
|
|
Lamp
shells |
|
Pseudocerotidae |
?Pseudocerus
|
lividus |
Flatworm |
|
|
|
|
Segmented
worms |
|
|
Eurythoe |
complanata |
Worm |
|
Nereidae |
?Perinereis |
amblyodonta |
Ragworm |
|
|
?Australonereis |
ehlersi |
Worm |
|
Serpulidae |
Galeolaria |
caespitosa |
Encrusting
worm |
|
Serpulidae |
Filograna |
implexa |
Tangled
tubeworm |
|
Aphroditidae |
Aphrodite |
australis |
Sea Mouse |
|
Arabellidae |
Arabella |
Sp. |
Worm |
|
Capitellidae |
Dasybranchus |
Sp. |
Worm |
|
Eunicidae |
Eunice |
antennata |
Worm |
|
Eunicidae |
Eunice |
aphriditois |
Worm |
|
Eunicidae |
Eunice |
australis |
Worm |
|
Eunicidae |
Lysidice |
Sp. |
Worm |
|
Eunicidae |
Palola |
Sp. |
Worm |
|
Lumbrineridae |
Lumbrineris |
Sp. |
Worm |
|
Polynoidae |
Lepidonotus |
Sp. |
Worm |
|
Sabellidae |
Branchiomma |
nigromaculata |
Worm |
|
Sabellidae |
Sabellastarte |
indica |
Featherduster
Worm |
|
Syllidae |
Syllis |
Sp. |
Worm |
|
Syllidae |
Trypanosyllis |
Sp. |
Worm |
|
Terebellidae |
Longicarpus |
modestus |
Worm |
|
Terebellidae |
Thelepus |
australiensis
|
Worm |
|
Terebellidae |
Terebella |
Sp. |
Worm |
|
|
2
unidentified |
species |
Ribbon
worm |
|
Goniasteridae |
Tosia |
australis |
Southern
Biscuit Star |
|
Asteropidae |
Petricia |
vernicina |
Velvet
Sea Star |
|
Asteriidae |
Coscinasterias |
muricata |
11-armed
Sea Star |
|
Asteriidae |
Allostichaster |
polyplax |
Many-armed
Sea Star |
|
Asterinidae |
Patiriella |
brevispina |
|
|
Asteriidae |
Uniophora |
granifera |
|
|
Goniasteridae |
Pentagonaster |
duebeni |
Vermillion
Star |
|
Echinometridae |
Heliocidaris |
erythrogramma |
Spiny
Urchin |
|
Cidaridae |
Goniocidaris |
tubaria |
Spiny Pencil
Sea Urchin |
|
Cidaridae |
Phyllacanthus |
irregularis |
Slate
Pencil Urchin |
|
Stichopodidae |
?Stichopus |
Sp. |
Sea
Cucumber |
|
Ophiomyxidae |
Ophiomyxa |
australis |
Brittlestar |
|
Ophiotrichidae |
Ophiothrix |
spongicola |
Brittlestar |
|
Ophicomidae |
Clarkcoma |
pulchra |
Brittlestar |
|
Aporometridae |
Aporometra |
wilsoni |
Crinoid |
|
Comasteridae |
Cenolia |
trichoptera |
Crinoid |
|
Palinuridae |
Jasus |
edwardsii |
Southern
Rock Lobster |
|
Portunidae |
Portunus |
pelagicus |
Blue Swimmer
Crab |
|
Dromiidae |
Dromidia |
Sp. |
Sponge
Crab |
|
Palaemonidae |
?Palaemon |
litoreus |
Palaemonid
shrimp |
|
|
?Leptomithrax |
gaimardii |
Spider
Crab |
|
Goneplacidae |
Plagusia |
chabrus |
Red bait
crab |
|
|
|
|
Hermit
crab |
|
Eriphiidae |
Ozius |
truncatus |
Reef or
Rock crab |
|
|
Caprella |
Spp. |
Amphipod |
|
|
Catophragmus |
Sp. |
Surf
Barnacle |
|
|
Balanus |
Sp. |
Barnacle |
|
|
?Chthamalus |
Sp. |
Barnacle |
|
|
?Tetraclitella |
Sp. |
Barnacle |
|
|
& many
other unidentified species |
|
|
LIST OF
MARINE PLANT SIGHTINGS AT PORT NOARLUNGA REEF (Part chart only)
|
Family |
Genus |
Species |
Description |
|
|
Cheilosporum |
elegans |
Encrusting
red alga |
|
|
Sporolithon |
durum |
Encrusting
red alga |
|
Rhodomelaceae |
Laurencia |
Sp. |
|
|
Rhodomelaceae |
Osmundaria |
prolifera |
Robust
red alga |
|
Ceramiaceae |
Wrangelia |
plumosa |
|
|
Ceramiaceae |
Ceramium |
Sp. |
Turf alga |
|
Gelidiaceae |
Pterocladia |
capillacea |
|
|
Gelidiaceae |
Gelidium |
Sp. |
Turf alga |
|
Rhodymeniaceae |
Rhodymenia |
australis |
Red alga |
|
Corallinaceae |
Metagoniolithon |
stelliferum |
Coralline
red alga |
|
Corallinaceae |
Corallina |
Sp. |
Turf alga |
|
Plocamiaceae |
Plocamium |
angustum? |
Foliaceous
red alga |
|
Cystoseiraceae |
Cystophora |
expansa |
Branched
brown alga |
|
Cystoseiraceae |
Cystophora |
intermedia |
Branched
brown alga |
|
Cystoseiraceae |
Cystophora |
monilifera |
Branched
brown alga |
|
Cystoseiraceae |
Cystophora |
moniliformis |
Branched
brown alga |
|
Cystoseiraceae |
Cystophora |
subfarcinata |
Branched
brown alga |
|
Alariaceae |
Ecklonia |
radiata |
Leathery
brown alga |
|
Stypocaulaceae |
Halopteris |
funicularis |
Foliaceous
brown alga |
|
Dictyotaceae |
Lobophora |
variegata |
|
|
Dictyotaceae |
Zonaria |
crenata |
Foliaceous
brown alga |
|
Dictyotaceae |
Zonaria |
spiralis |
Foliaceous
brown alga |
|
Dictyotaceae |
Zonaria |
turneriana |
|
|
Dictyotaceae |
Dilophus |
fastigiatus? |
|
|
Dictyotaceae |
Dilophus |
gunnianus? |
|
|
Dictyotaceae |
Dilophus |
marginatus |
|
|
Dictyotaceae |
Distromium |
flabellatum |
|
|
Dictyotaceae |
Dictyota |
alternifida? |
|
|
Dictyotaceae |
Dictyota |
dichotoma |
Membranous
brown alga |
|
Dictyotaceae |
Dictyota |
diemensis |
|
|
Dictyotaceae |
Dictyota |
naevosa? |
|
|
Dictyotaceae |
Lobospira |
bicuspidata |
Foliaceous
brown alga |
|
Dictyotaceae |
Dictyopteris |
australis |
|
|
Dictyotaceae |
Dictyopteris |
muelleri |
|
|
Sargassaceae |
Sargassum |
decipens |
Branched
brown alga |
|
Sargassaceae |
Sargassum |
spinuligerum |
Branched
brown alga |
|
Sargassaceae |
Sargassum |
fallax |
Branched
brown alga |
|
Cladostephaceae |
Cladostephus |
Sp. |
Foliaceous
brown alga |
|
|
Bryopsis |
plumosa? |
|
|
Caulerpaceae |
Caulerpa |
annulata |
Foliaceous
green alga |
|
Caulerpaceae |
Caulerpa |
brownii |
Foliaceous
green alga |
|
Caulerpaceae |
Caulerpa |
cactoides |
|
|
Caulerpaceae |
Caulerpa |
flexilis,
var. muelleri |
Foliaceous
green alga |
|
Caulerpaceae |
Caulerpa |
simpliciuscula |
|
|
Caulerpaceae |
Caulerpa |
trifaria |
Foliaceous
green alga |
|
Codiaceae |
Codium |
pomoides |
Lumpy green
alga |
|
Codiaceae |
Codium |
duthieae |
|
|
Codiaceae |
Codium |
perriniae |
|
|
Codiaceae |
Codium |
capitulatum |
Turf alga |
|
Ulvaceae |
Ulva |
lactuca |
Membranous
green alga |
|
Anadyomenaceae |
Struvea |
plumosa |
|
|
Siphonocladaceae |
Dictyosphaeria |
sericea |
Lobed
green algae |
|
Valoniaceae |
Valonia |
Sp. |
|
|
Cladophoraceae |
Chaetomorpha |
aerea |
Green
alga |
|
Cladophoraceae |
Cladophora |
bainesii |
|
|
Cladophoraceae |
Cladophora |
coelothrix |
|
Sepia apama, the giant Australian
cuttlefish, in Whyalla, S.A.
by Evan John
One of
the most spectacular events of the natural marine world takes place annually between
May and August in the reef areas of upper Spencer Gulf, around Black Point,
Point Lowly and Fitzgerald Bay, north of the town of Whyalla, on Eyre
Peninsula, South Australia.

Fig. 1. Sepia Apama
It is here that the giant Australian
cuttlefish, Sepia apama, migrate in the thousands to mate and spawn. The
low rocky reef areas provide a hard rocky surface on to which the cuttlefish
can attach their eggs, for much of the rest of upper Spencer Gulf is sand, sea
grass flats and mud banks. It is an amazing spectacle, as these unique marine
animals can be observed changing colour, shape and texture, and performing
mating rituals. It is believed that this kind of aggregation occurs nowhere
else in the world in such numbers.
Sepia
apama is the largest species of marine animals commonly called
cuttlefish; it is believed that specimens as large as 1.5 metres have been
recorded. S. apama is a mollusc –
and is, therefore, a closer relative to
the common garden snail, rather than to its marine compatriots, the
fish. It belongs to the Class Cephalopoda, a group which presents a complete
contrast to the majority of the molluscs in habits, and in many respects,
points of organization. Cephalopods have the power of rapid movement, no
external shell, (although they do have an internal supportive structure, (the
cuttlebone or gladius), and a circle of four pairs of fleshy arms and two
elongated tentacles that surround the mouth, which contains a pair of mandibles
or “beaks” somewhat similar to parrots. They also have a relatively
well-developed nervous system, and their eyes are quite specialized, in that
they have a lens, cornea, retina, and distinctive “W-shaped” pupils. This
suggests that the eyes are used for observation as well as just transmitting
light sensations to the nervous system like snails. Vision is believed to be
highly developed, rivalling that of humans, and divers have described meeting
the gaze of a cuttlefish as one which is that of a highly intelligent creature
“in there looking back”. Cuttlefish and octopus are used in medical eye
research because of the similarity to the human eye.
The word
Cephalopod is constructed from the Greek words kephale (head) and podos (foot).
This conveys the notion that the limbs emerge from the face, which, in effect,
is what happens. Cuttlefish have a body shape something like a flattened
football, with eyes and arms and tentacles at one end, and a “fin” which runs
along each side. This lateral “fin” undulates, and propels the animal through
the water. In addition, the cuttlefish “bone”, (often washed up on the beach
and used as a source of beak strengthening and diet calcium for caged birds,)
is honeycombed with gas-filled cells, producing neutral buoyancy, thus allowing
precision and delicacy of movement when desired. When rapid propulsion is
necessary, the animal is able to draw water into the mantle cavity, and force
it out through a “nozzle” or siphon in the side of the body underneath the eye.
This siphon can be swivelled to change direction, and relaxed or constricted
when control of speed is desired.
Probably the cuttlefish’s most fascinating behaviour is its ability to change
colour. There seems to be a variety of stimuli which start this amazing
display, ranging from movement between light and dark areas, an apparent
attempt to hide and camouflage, and probable mating activity. On some
occasions, waves of coloration changes pass over the length of the body, in
less than a second, moving from head to rear, probably associated with a
warning display. Colour variation is due to a system of chromatophores, minute
multi-nucleate pigmented cells, red, brown, yellow or blue, embedded throughout
the animal’s skin. These are surrounded by small radial muscles, connected to
the central nervous system. When the muscles contract, the cell expands, and
the pigment it contains becomes more apparent. Chromatophores are organized
into three layers, each containing a different pigment colour. The ability to
manipulate each layer independently means that quite a large range of colours
are possible as a result of the blending effect of the different pigments. This
colour composition is enhanced by the presence of small cells called
iridocytes, that lie above and below the chromatophores, creating a mirror-like
effect. It is incredible to watch a cuttlefish rest in a shaft of light – the
part of its body in the light changes quite quickly to a distinct, patterned
colouration, whilst that in the dark stays a continuous duller colour.

Fig 2 Male cuttlefish displaying
In
addition, these remarkable creatures can change the texture of their skin.
Small wart-like protuberances called tubercles on the skin can be made to
expand and contract, resulting in the skin of the body becoming more “bumpy”
when against a background of marine plants like Caulerpa or Ecklonia
or Hormosira.
As
previously mentioned, the area around Whyalla is quite unique because it is one
of the relatively few areas in southern Australia where S. apama
congregate to mate and to spawn. During mating, males and females lock
tentacles, and the male uses one of its longer tentacles to transfer a packet
of sperm into the body of the female. She then lays between 100 and 300 eggs,
attaching them under a rock or to the roof of a cave in the shallow reefs or
some other such sheltered location. It is believed that the species takes about
four years to reach sexual maturity.

Fig. 3 Cuttlefish eggs
After
mating, both male and female animals become lethargic, their appearance takes
on a greyish, worn and tattered look, and they then die.
Cuttlefish are an important link in
the food chain. From stomach analyses, it seems that they are a prime food source
for snapper, yellow-tail, dolphins and sea birds. Research also shows that
cuttlefish make up a significant proportion of the diets of Australian fur
seals and sea lions. There is little doubt that
their biomass component is critical to maintaining viable breeding populations
of animals at the upper end of the marine food chain, in marine ecosystems of
which they are a part.
Fortunately,
up until about 1996, cuttlefish fishing was pretty much restricted to bait
fishing by local fishermen, with some recreational catching and a very limited
commercial catch. However, in 1997, commercial markets were established in S.E.
Asia, and the recorded catch was 255 tonnes, or over 250,000 cuttlefish, by
relatively few boats. Commercial exploitation of this resource was at the time
unrestricted and unmanaged.
In 1998, there was a doubling of the fishing effort, with many more boats and
fishermen per boat! At the beginning of the season in early May, fishermen were
ready for the cuttlefish to arrive, and within four weeks had so reduced the
stock that they voluntarily stopped fishing for 10 days to allow stocks to
recover. By early June the catch was so low that the fishermen had stopped
fishing in any significant numbers. Despite this self-imposed moratorium the fishing
did not improve to any noticeable degree, and it was at this point, with
ongoing lobbying by a number of organizations, that the then South Australian
Government announced the closure of the fishery until September 1998, whilst at
the same time initiating a three year study of the cuttlefish population, to
determine the effects of commercial fishing and the possibility of sustainable
exploitation in the long-term. Researchers at Melbourne University, who had
studied Whyalla’s cuttlefish aggregation over two seasons, had written to the
Premier of South Australia in 1998 pointing out that “the rapid recent rise in
exploitation of this spawning aggregation is likely to destroy this unique
natural event before the impacts of this harvest are fully understood.
Cuttlefish catches from this small area of rocky reef have risen unchecked from
negligible levels to more than 200 tonnes per year in less than three years.
More than a quarter of a million cuttlefish were pulled from this small, region
last year (1997). Signs of collapse are already evident this year …".
As a
consequence, responding to many expressions of community concern, a Sanctuary
near Point Lowly was set aside to protect some cuttlefish stocks.

Fig. 4 Cuttlefish mating
Unfortunately
the area of the Sanctuary contained large areas of sandy beach, quite
unsuitable for cuttlefish to breed.
In
February 1999, the South Australian Government made the decision to close the
local cuttlefish spawning grounds and stop commercial fishing during the 1999
and 2000 seasons, so further research on Sepia apama could be undertaken
by the South Australian Research and Development Institute (SARDI).
Anecdotal
weekly observations by the divers during the 2000 season indicated that the
biomass had not recovered at all in the two and a half years that the spawning
grounds have been closured.
That this
unique congregation of Sepia apama must be protected from commercial
exploitation appears obvious for at least two sound biological reasons:
Taking
the animals as they arrive at the breeding site gives them no time to mate and
spawn, hence limiting future population numbers.
Given the
short life-span and low reproductive rate, (they lay relatively few eggs), and
the disproportionately high predation rate of young cuttlefish, current stocks
may well be unsustainable.
There are
other valid reasons why the nurturing of current stocks of S. apama at
Whyalla is essential.
There is significant scientific and education interest.
SARDI research to date, the lack of other data and
research material on
the animal, and the
acknowledged limitations of
the scope of the current research work,
make it clear
that there will be insufficient information to make
informed and safe
management decisions on this
resource from the point
of view of allowing
any form of commercial exploitation. In addition, there
has been, and
continues to be, unprecedented interest from
the scientific and documentary film - making communities, research
groups and media from
around the world.
The animal’s
behaviour is unique, recognized by
marine scientists both
nationally and internationally. The site also has substantial existing
value for research and
monitoring, and is unique for its
accessibility and scale.
S. apama is
ecologically significant – in the marine food chain of S.A. waters
There is substantial potential for sustainable
eco-tourism
Divers from across Australia, North America, Chile and Europe have
travelled to Whyalla for no other reason than to dive with the cuttlefish –
regarded as the “chameleons” of the sea. Feedback from these visitors suggest
that they are the forerunners of thousands of diving enthusiasts who will
travel around world to experience this unique marine event.
The animals are vulnerable to fishing
Permanent
protection is essential for the animal’s survival.
It is
well worth taking the effort to visit Whyalla during the breeding season of the
giant Australian cuttlefish, Sepia apama.
Local
diving and boating organizations run charters to the area, enabling divers and snorkellers to witness this unique phenomenon.
Author’s note
I must acknowledge the assistance of two extremely dedicated people, for
their comments, thoughts and material in my preparation of this article:
Tony Bramley, of the Whyalla Dive Shop, who, with his diving
colleagues, has worked tirelessly with local and overseas scientists and the
S.A. Government, to ensure the
protection of the cuttlefish breeding grounds, and Ron Hardman,
who has recorded cuttlefish and their activities in a series of brilliant
copyrighted photographs and videos. He
has very kindly allowed me the use of some of his photos in this article.

Giant
Cuttles - Photographer: Paul Macdonald, October main from the MLSSA 2007
calendar.
Fossil Cave / Green Waterhole Cave (5l81)
Bone Retrieval Dives for Dr Trevor Worthy
(University Of Adelaide), 27th/28th May 2006.
by Dave Albano/Peter Horne
supplied by Neville
Skinner
PARTY
Peter
Horne (Team Coordinator), Neville Skinner
(underwater photographer/ support & safety Diver), David Albano
(support/safety diver) and Mark Nielsen (safety diver); Ian Lewis
(surface support).

Carrying gear down to the cave’s
lake which is situated in the dark alcove behind the two scuba cylinders at the
far end of the collapse (Peter Horne).
OBJECTIVES
To attempt to
relocate the 1979 Flinders University survey star-dropper posts along the “N”
line, especially N3 dropper, and an adjacent labelled tag known as “Aslin Site
12” (“Site 07” during the 1979 project); to attempt to photograph the area
before, during and after any bone-digging work; and to collect samples of bones
and sediment around Tag 12.

Left: The research party:
Neville Skinner (left rear),
Ian Lewis (centre front),
Mark Nielson (right rear).
Right: And the authors,
Dave Albano/Peter Horne
(left to right).
OUTCOMES
Dive
One: Saturday 27th May 2006 (duration
approx. 45 minutes).
Peter
and Neville descended first through the “Letterbox” with a large open reel of
thick white synthetic rope with the intention of locating and securing the old N3
star-dropper. The water was noticeably
dark and murky, suggesting that crushed grass observed around the carpark area
was most likely caused by a group of other divers earlier that morning, which
was unfortunate from the point of view of photography. The water level was also lower that Peter had
ever seen, and there was a substantial air section extending into the
normally-flooded ceiling area of the cave.
Their first
observation during the descent into the gloom was that none of the marker tags
remained on the star-droppers which were located, and many of the droppers had
either disappeared or fallen over (some were later found to have corroded right
through at their bases, leaving just a rust-filled hole in the rock). The lines
which had previously linked the main droppers together were also missing and it
took around 5 minutes before the correct line of droppers was relocated. As Peter approached what he believed was the
N3 dropper he was pleased to see the yellow Site 12 marker floating off the bottom
exactly where it was expected to be, despite the 18 years which had passed
since the last mapping project he had coordinated there back in 1988.

Running the thick white safety/reference line down into the main chamber
along the original “N” line (Neville Skinner).
Peter tied the
white line to N3 and proceeded to assess the area while Neville took photos,
and because he only had some small helmet-lights for illuminating the scene,
David and Mark supplied additional side-lighting for this task. A fallen dropper (believed to have been N1 or
N2) was found rusted through at the base and leaning against the back wall,
just over a small deeper hole, and Peter collected some of the more obvious (and
smaller) bones and carefully scraped samples of the sediment into a 2-litre
plastic ice-cream container using its flat lid, minimizing hand contact as much
as possible.

Tying off the white
reference/safety rope to (presumed) star-dropper N3, about 1.5m beyond which
Tag 12 can be seen floating in a small area of flatter calcite-covered silt
(Neville Skinner).
The first
container (#1, blue) was used to hold material which was collected from within about
2-3 metres of Tag 12 (around 9 metres depth), with some slightly shallower
samples included. No obvious bone
material was seen below Tag 12 and the floor in this area basically comprised a
2-3m wide flatter section of boulder, with a fairly thick deposit of calcite
rafts.

Collecting sediment and bones (Neville Skinner).
Container
No. 2 (gold) was used for material which was collected considerably shallower
than the Tag 12 location, virtually directly above and over the boulders at a
depth of about 6 metres close to the N4/N5 pegs (visible in the photo below),
after Neville had located a partial skull in the silt there.
Container No.
3 (blue again) was used to store more bones which were also within a 3-4m
radius of N3/Tag 12, and bones found included many thin, long bones and a few
larger ones including two which are obviously from large mammals such as
kangaroos.


Sample 2, using a yellow container, near N4/N5 (pegs visible near
boulder in top photo – Neville Skinner).


Sample 3 (blue container) to the right of Tag 12, and placing the
samples into the wire bone basket for transporting back to the surface. Note the reduced visibility (Neville Skinner).
This first
dive was disappointing to some extent with regards to the poor visibility and
the scarcity of good bone-fields near Tag 12, but it was a safely-executed and
interesting dive nonetheless and provided important preliminary information for
possible future work, which should ideally include more photography and
silt/bone-collecting around N3/Tag 12 as well as sediment sampling directly
below Tag 12 (at the wall/floor interface) and a closer exploration of the
deeper small holes in that area which escaped earlier mapping documentation.
Dive
Two: Sunday 28th May 2006 (duration
approx. 45 minutes).
The dive party
comprised the same divers as the previous day, but Ian was not in attendance
this time. The weather was atrocious but the water was spectacularly clear, and
it was a very easy task to relocate Tag 12 (a standard cave diving line/reel
was used this time instead of the thick white rope). Peter descended first with David and both
divers collected more material/samples around Tag 12, storing their samples in
three white ice-cream containers which like the smaller ones of the previous
day, were carried down to the site in a bone-collecting basket (wire cage with
split-pin lid, used during the original research project by SAUSS Inc). Container No. 4 (marked “Pumpkin Soup” –
hopefully nobody will believe the label!) was taken down to about 11 metres
where a small area of wall/floor interface had collected some sediment, which
was carefully scooped into the container.
This was below and slightly to one side of the Tag 12 area of interest –
any material which had fallen straight down from Tag 12 would likely have
fallen down through some small deeper holes which require further investigation
with single or side/mount scuba cylinders or the like. Container No. 5 (unmarked) was basically
another general sample close to the previous day’s #3 container collection on
the flat calcite area to the right of Tag 12, and Container No. 6 (marked “Soup
5/7/00”, see above warning!) was at a depth of 6m where David had spotted some
interesting skeletal bits and pieces under the edge of a slab. During this collection Peter realised that a
fallen, nearly-buried dropper there still had the original tag N4 loosely
attached; this was removed and placed in the container to assist later
labelling.


Collecting bones and sediment from along the edge of the boulder near N4/N5;
the unlabelled standing dropper is N5, and N4 can be seen lying to its right
(Neville Skinner).
At
various times during this dive Neville took more photos, and after the samples
had been taken to the surface Neville took Peter and Mark back down to the deep
floor-hole area to show them additional cavities and bones which await
collecting and recording in the future.
At the conclusion of the diving and collecting activities the larger
bones were wrapped in wet newspaper (in hindsight, not a good idea for possible
DNA studies, in view of the paper and ink base, but that’s how the earlier
Flinders teams did it) and the other containers were padded and stacked in a
plastic Esky to keep them cool and protected for the journey back to Adelaide
University, where they were delivered by Peter to Dr Worthy on Monday 29th
May 2006.
Preliminary
assessment indicated that some bones could be of considerable interest and
hopefully further more detailed research may come from these efforts in the
not-too-distant future.
Peter
Horne, Team Leader (former Projects Coordinator, South Australian Underwater
Speleological Society Inc; Project Leader of SAUSS Project No. 1, Fossil
Cave, 1988 and former Manager of Mapping & Research, Cave Divers Association
of Australia Inc.)
What You Should Know About Great White Sharks (DRAFT)
by Phil Kemp
INTRODUCTION
In
2005, one fatal shark attack and two non-fatal attacks once again shocked the
South Australian community. The information I have prepared here will give you
an insight into the behaviour and habits of the Great White Shark and our need
as a community to embrace its survival, rather than call for culling of the
species after each attack, which could very well lead to its extinction.
There
have been many headline-grabbing descriptions in recent years that portray the
Great White Shark as a “killer”, a stereotype that many researchers will argue
against and suggest that this title is quite undeserving. As most of us have
experienced throughout our lives, we are often fearful of things we do not
understand and the things we don’t know. To gain an understanding of the Great
White Shark can assist us in respecting these creatures rather than fearing
them.
They
are in danger of becoming extinct and, as I will explain further throughout
this article, to gain a deeper understanding we need to view the Great White
Shark differently.
It
may be surprising, considering the fearsome reputation of a Great White Shark,
that they are listed on the IUCN Red List of Threatened Species.1, 2, 6
and 7 This fact may seem incomprehensible to some people as the Great
White Shark is commonly portrayed as a fearless mindless killing machine.
Also
included in this article are some facts, statistics and behavioural information
about the Great White Shark. You will also learn that Great White Sharks are
intelligent and complex animals, supremely adapted to their environment. Each
individual appears to demonstrate distinctive behavioural characteristics and
appears to have some learning capacity. 2 and 6 Despite its position
as an apex predator (top of the food chain), the Great White Shark faces
numerous threats in its environment, just like every other high order carnivore
and just about any other large wild animal in the world. To ecologists, this is
not surprising at all. There’s also a section included in this article on
methods that may assist in reducing the risk of being attacked.
Even
though the Great White Shark is endangered and needs our help to survive in to
the future, so do many other living creatures in the marine eco-system. These
other creatures depend on the Great White Shark’s continued survival and
predominance.
So
what possibly could be threatening them? The answer is not that surprising, we are!
You can help save the Great White Shark, however, and, by doing so, aid in
preserving the balance of our local marine eco-system.
So
please read on and let us open the window into their world and learn how you
can play an important part in its survival and everything that lives in its
liquid universe and, at the same time, and most of all, be safer.

FAST FACTS ABOUT THE
GREAT WHITE SHARK
The Great
White Shark is scientifically known as Carcharodon carcharias. It is, however, most commonly known
around the world as the ‘Great White’. Here in Australia it is also known as
the ‘White Pointer’.
Shape & Colour
The Great
White has a torpedo shaped body and conical snout. Its colouration is a bronzy
and greyish brown on top (dorsal surface) and white underneath (ventral
surface).
The number,
position and shape of fins is distinguishing and aids in shark identifications.
Great White Sharks have a large first dorsal fin and very small second dorsal
fin, a pair of large pectoral fins and a vertical crescent shaped tail fin
(with the dorsal lobe being only slightly larger than the ventral lobe). It
also has five large gill slits extending along each side of the throat, and
large dark eyes.
The
Great White Shark has large triangular serrated teeth. They have 50 individual
teeth positions in their jaws, with 26 in the upper jaw and 24 slightly
pointier teeth in the lower jaw. Each of these positions may have 1-3 row(s)
exposed with several other rows being developed from within the gum and
awaiting to move forward as the teeth need replacing (like a conveyer belt).
Size
Nobody
knows for sure exactly how big the Great White grows. There has been a lot of
debate and a lot of unconfirmed wild claims of Great Whites measuring up to 7m
in length and up to 3,000kg in weight being captured. It is generally accepted,
however, that the maximum length of this species is between 5.5m to 6m, with
the female being larger than the male when fully matured. There have been
dependable reports of captured Great Whites weighing in above 2,000kg, but
generally not much more than 2,500kg. 1, 2, 6, 9 and 10
Diet
Great
White Sharks feed on a diversity of prey items ranging from snapper, tuna and
squid to larger mammals like seals and sea lions, dolphins, whales, stingrays and
other sharks. Hence, our presence in the sea means an attack is a possible
consequence, albeit an unlikely one in most circumstances.
Habitat
Great
White Sharks are uncommonly encountered, yet widespread throughout the world’s
temperate oceans, preferring cool temperate waters generally ranging from 12oC
to 22oC, and between 60o latitude North and 60o
latitude South (Feurguson, 1998). 2, 6 and 9 Their few remaining
population strongholds seem to be in Southern Australia, South Africa and Western
North American waters.
Current
satellite tagging information shows that many of the Great White Sharks spend a
proportion of the year in the pelagic zone, away from the continental shelf.
Currently, knowledge of what sharks do out there is limited. It is important,
however, for us to find out why they do this, to understand their lifestyle,
and to help save the Great White from extinction. The relatively small
knowledge of Great Whites means that continued scientific research is
imperative to help preserve the Great White and assist in recovery from the
threatened status that this species holds. 1, 2, 6, 9 and 10
Abundance
There
is no accurate estimation on the numbers of Great White Sharks both in
Australian waters and around the world but real evidence and reports show that
their numbers have declined since mid last century.1, 2, 6, 9 and 10
There is no
evidence that numbers have significantly increased in recent years as is
reported widely by the media seemingly any time that a shark attack occurs.
Reports have shown that fewer Great Whites are being captured each year in
recent times than a couple of decades ago, which may suggest a decrease in
numbers. Plain logic, however, also suggests that such a slow breeding animal
cannot possibly rebound significantly in the amount of time that the Great
White has been protected here. Statistics also indicate that we are not seeing
a trend of increasing shark attacks against a trend of an increase in the human
population. 3
Predators
Great
White Sharks have few natural predators. It has, however, been documented that
they have been attacked by Killer Whales. 1, 2, 5 and 8 It is humans
though who pose the greatest threat to the Great Whites. This is a result
through a number of activities, which will be discussed further in more detail
later on.
BIOLOGY OF THE GREAT WHITE SHARK
Here
are just a few unique and interesting facts about the Great White Shark: -
(It
is important, however, to note that there are over 450 recognised shark species
that all share some common characteristics.
For
example:
- Their senses, i.e. sight,
smell and the ability to detect low frequency vibrations and
electro-magnetic fields.
- A skeleton made out of
cartilage rather than bone.
- Their skin, covered with
tiny tooth-like scales that help protect their skin and help to reduce
drag.
- Large livers that are rich
in low-density oil, which helps to prevent them from sinking, in the
absence of a swim bladder that’s present in bony or scale fish.)
The
Great White has evolved an ability that very few species of sharks are able to
do and that is to increase their body temperature up to 14oC greater
than the surrounding water through the process of heat exchanging. This gives a
predatory advantage in colder water compared to other sharks, which require
warmer or tropical waters to maintain physiological function. Great White
Sharks are closely related to two mako species of sharks. Other shark species
including the Salmon Shark, Porbeagle and at least several species of Thresher
Sharks can do this as well.

Great
White Sharks can hold their heads above water and have the ability to focus
their eyes with special muscles for above water focus
Great
White Sharks have the biggest olfactory lobes of all sharks, providing the
greatest sensitivity to smell. This is possibly also for social reasons rather
than just detecting a dead whale, a seal or sea lion at vast distances. Great
White Sharks have a highly developed visual sense with great colour vision.
Sharks have electro-receptors called ‘Ampullae of Lorenzini’ which give them the ability to
detect electrical fields. The Hammerhead Shark is believed to have the
most heightened of this sense, but the Great White Shark still can detect down
to 125 millionths of a volt.1, 2 and 6 All sharks have another
method of detecting prey - the ‘Lateral Line,’ which extends down each
flank of the shark, detects vibrations in the water. 1, 2 and 6

Ampullae
of Lorenzini
With the complex sensing organs the Great White
possesses, it takes a relatively large well-developed brain to coordinate these
effectively. Combine this with its complex social structure, (which has just
recently been observed by researchers in the wild), the ability to adapt new
strategies for catching food and the ability to learn from past experiences,
would indicate relatively higher intelligence than we have previously given
them credit for.
GREAT
WHITE SHARK BEHAVIOUR
It has long been thought that the Great White
Shark has been a solitary hunter. Extensive studies in recent years have
discovered a complex social behaviour and dominance hierarchy status in the
Great White when they gather around food sources.
Researchers have so far managed to distinguish
ten different rituals and displays that the shark uses to settle differences in
most cases, demonstrating dominance through posture and body language to
challenge each other, rather than using violent force.2 and 6
Scientists have also witnessed behaviour in the
Great White Shark, which leads some of them to think that they are curious
creatures as well. They have displayed an inventive way of manipulating objects
such as biting, balancing, chasing, and hitting objects like five-gallon drums,
etc.. The Great White tends to have a sense of play with living and non-living
things, giving the impression that they are not always interested in them as a
food source.2 and 6
Great White Sharks tend to use a range of
investigative skills using their snouts and highly sensitive sharp teeth to test
an object, as opposed to a creature that can touch or examine an object with
its hands (like us humans), paws or feelers. Researchers have witnessed this
type of test biting, along with bumping objects, and it is believed to be
assisted by special types of touch receptors on their ‘face’.
It is also known that in some of the attacks on
humans where the victims have not sustained serious injury, the shark has had
just one bite and then released the victim, supporting the knowledge that
sharks will test or examine a subject and release, rather than attempt what
most people would believe to be a feeding frenzy.
It could then be said, when it comes to items
on their menu – we are not on it! The question that still remains is “why are
we being targeted for occasional attacks?”
A simple explanation could be put down to test
biting for palatability (to see if we have enough fat/energy content) and to
give an indication of whether or not the object can be consumed easily. The
next step is to test to see if the shark is prepared or motivated to go to a
higher level of energy expenditure to consume the target. So the good news is
that we do not normally have enough fat/energy content for them to want to
consume us. At the same time, however, it could be a territorial attack to keep
other large competitors out of its immediate area by claiming the victim.
Whether or not we can prove that Great White Sharks mistake us for seals (while
we float on the surface) from visual cues has led to debates amongst scientists
and researchers. The fact that we are rarely attacked when sharks have the
ability to see us, however, gives credence to the fact that they have excellent
vision.1, 2 and 6
The knowledge and background we have on shark
attacks suggests they are not the malicious killers that have been portrayed in
movies. Knowing this, however, does not reduce the concern of the community and
those who have had the misfortune of being attacked. Everyone should be aware
of safety in the water and being aware of hazards in relation to shark attacks.
(See the section further in this information for more helpful hints).
SHARK
ATTACKS
Psychologists have found out through research
and extensive testing that no other word in the English language strikes more fear
in us than the word ‘shark’. This may be because when we enter their liquid
environment, which is so alien to our terrestrial senses, we are rendered
virtually helpless.2 and 6 To be safer, we have to learn and
understand how they behave and what measures we have to take to remain safer so
that we still can enjoy ourselves and share their world.
Following recent events, it is easy to draw the
conclusion that shark attacks are on the rise. Statistics, however, do not
support this view. As the worldwide human population continues to rise year
after year, so does our interest in aquatic recreation. The number of shark
attacks in any given year or region is highly influenced by the number of
people entering the water. Note the nearly identical increase in beach
attendance, drowning rescues, and shark attacks. In comparison, you are still
far more likely to be killed in a car accident than attacked by a shark.
Statistics from the ASAF show that there has been only an average of 1.3 fatal
shark attacks per year in Australia over the last 20 years. Significantly more
people are attacked each year by other animals. There has been for example: -
- 2-3 fatalities per year due to
bee stings.
- Over 300 people a year in
Australia are bitten by snakes, with an average of 3 fatalities as a
consequence.
- Four crocodile attacks in the
top end of Australia last year (as of September 2005) and there were at
least three in 2004.1, 3 and 4
When we discuss why a shark attacks humans, we
must think of the motives of the shark. Our ability to think clearly and
rationally when we face the terrifying thought of being eaten alive will likely
overwhelm any thought of clear thinking. Being educated and having a clearer
understanding of the motives of the shark attacking us may assist that thought
process when we discuss the issue of being attacked.
The majority of attacks on humans are
unprovoked and may be more exploratory in nature, motivated by the Great White
Shark being inquisitive rather than being motivated by hunger.
|
Australian Bureau of
Statistics Fatal
Accidental Drowning & Submersion from 1994 & 1995 in the following
categories |
ASAF
Recorded |
|||||
|
Year |
Surfboard
Riding |
Rock
Fishing |
Skin
Diving |
Drowned while swimming at an ocean beach, a river, lake, & SCUBA
harbour, estuary, bay, or lagoon. |
Fatal attacks by Great White Sharks |
|
|
1994 |
3 |
14 |
27 |
79 |
Total 123 |
|
|
1995 |
2 |
13 |
14 |
68 |
Total 97 |
|
|
1876-2005 |
|
|
|
|
|
Total 39 |
The
Australian Bureau of Statistics provides evidence of more humans losing their
lives in other water activities than due to Great White Shark attacks.
The majority of unprovoked attacks are relatively
gentle compared with the outrageous damage these huge and powerful predators
are clearly capable of inflicting. In attacks off the South African coast, of
63 cases, 29 (or 46%) of the victims were bitten but sustained no tissue
loss whatsoever.2
The remaining minority of unprovoked attacks
are generally more aggressive and may be a case of mistaken identity,
especially if the water’s visibility is reduced significantly due to poor
conditions.
A 1981 paper written by Ralph Collier and
Daniel Miller says “numerous accounts and historical evidence suggests that
in an unprovoked attack the shark will remain in the area and attempt to
inflict further bites if the person fights back in the initial attack.” 2
With this in mind, the Australian Shark Attack File suggests that some methods
of repelling sharks may have different outcomes every time. It all depends on
each individual situation and with the conditions and the size of the shark.3
In conclusion, humans kill far more sharks each
year than sharks kill humans. Statistically, you are even more likely to be hit
by lightning than attacked by a shark.
Total Shark Attacks in Australia by ASAF
(Not every
shark attack was by a Great White Shark)
|
State |
Total Attacks |
Fatal Attacks |
Last Fatal Attack (up to 2005) |
|
NSW |
243 |
72 |
1993 Byron Bay |
|
QLD |
223 |
71 |
2004 Opal Reef |
|
VIC |
34 |
7 |
1977 Mornington Peninsula |
|
SA |
49 |
21 |
2005 Glenelg Beach |
|
WA |
71 |
13 |
2005 Houtman Abrolhos Is. |
|
NT |
12 |
3 |
1938 Bathurst Island |
|
TAS |
21 |
5 |
1993 Tenth Island, Georgetown. |
|
Total (As of Sept 2005 for all Australian States Combined) |
653 |
192 |
|
1876-2005
ASAF Recorded Attacks by Great White Sharks in Australia (N=76)
|
State |
Total Attacks |
Fatal Attacks |
Last Fatality |
|
SA |
27 |
15 |
2005 |
|
NSW |
23 |
11 |
1993 |
|
VIC |
10 |
3 |
1956 |
|
WA |
14 |
5 |
2005 |
|
TAS |
10 |
4 |
1993 |
|
QLD |
2 |
1 |
1992 |
|
Australia in Total |
86 |
39 |
2005 |
For
more detailed information on shark attacks and statistics please visit www.sharkfoundation.com or www.zoo.nsw.gov.au
WHY THE GREAT WHITE SHARK IS AN ENDANGERED SPECIES
The Great
White Shark is endangered because they grow and mature slowly and because they
are believed to reproduce once every 2-3 years, producing only a small litter
each time. These characteristics make the Great White Shark highly vulnerable
to even moderate rates of removal from the breeding stock. Natural mortality
plays its part in the population stability of Great White Sharks, keeping the
numbers down like it does with every other living creature. Great Whites have
very little to fear from other marine life, with the rare exception of the
Killer Whale. Attacks by Killer Whales for example, although rare, are possibly
to protect younger members of the pod from any potential threats and to remove
competition. There could possibly be a few reasons why the Killer Whale
would attack a Great White. One reason could be for the shark’s large liver,
which is rich in oils and would possibly make a tasty snack for the Killer
Whale. A second reason would be for the Killer Whales to protect their young
from the possible threat by a Great White Shark in the area.

Notice
the damage to the gills caused by another Great White, which nearly proved
fatal
Even though
Great Whites settle most of their differences through posture display and
rituals, as mentioned before, not all conflicts, however, are resolved
peacefully. The biggest threat to Great White Shark numbers, however, is
humans. Every mature Great White Shark removed from its environment represents a
significant loss to the marine ecosystem.
So how is this
possible? There are numerous ways for the cause of their depletion.
Commercial
Fishing
Fishing
is currently the largest cause of Great White Shark fatality, predominantly
resulting from by-catch. ‘By-catch’ means that
even though they are not targeted, they still have a tendency to get caught up
in fishermen’s nets and hooked on long-lines when trying to catch the same
school of tuna that the fishermen acquire. They also try to scavenge from fishermen’s
nets once the fishermen have made their catch and get entangled in the nets
during the process. In South Australia, estimations of around 10 to 100 are
killed due to this according to designating reports. As Great White Sharks are
inquisitive and approach boats and objects, this increases the likelihood of an
incidental entanglement in fishing equipment.
Through
recent studies conducted by the CSIRO Marine Research team tagging and
satellite tracking Great White Sharks, the scientists have discovered that
Great White Sharks may follow similar paths when in travelling mode from one
destination to another. If the scientist can identify clear highways that the
Great White travels, then maybe commercial fishing companies could possibly
avoid fishing in these areas.
Illegal Trading & Sports Fishing
Great White Sharks have been
protected in Australia since 1998 and are now CITES (Convention on
International Trade of Endangered Species) Appendix II listed. There has been no
illegal trade, or very little, for shark fins on the black market since. Sports
(recreational) fishing for trophy purposes (for Great White jaws and teeth) has
been very popular, especially after the movie ‘Jaws’. Now that they are
protected, however, it’s illegal to hunt Great Whites and there are heavy
penalties that apply for anyone found guilty of taking a Great White.
Penalties include a maximum $100,000 fine under fisheries regulations but
generally, for a first offence, this may involve a $4000 fine and/or one year’s
gaol. For a second offence, this may involve a fine up to $8,000 and/or 2
year’s gaol. 1, 7, 9, 10, 12 and 13
WHAT WOULD HAPPEN IF THE GREAT WHITE SHARK BECAME EXTINCT?
No one knows
for sure exactly what would happen if they did become extinct. What is known
from many scientific studies and models, however, is that the removal of the
top-level predator wreaks havoc on their entire ecosystem. How is this
possible? Well, because Great White Sharks are top order carnivores, they
directly influence the abundance and diversity of all other populations in
their environment.
For
example, when lower middle-level predators, like seals, dolphins and large fish
species, are not checked and balanced in our local environment, this would
result in population collapses down the line, leading to some populations
replacing others and a less rich and diverse environment ensuing. If this
happened, this would have a certain impact on our local commercial fisheries as
well.
As you
can see, Great White Sharks are far more valuable alive than dead. So it’s up
to us to do something, as we are the ones with the power to stop this, just as
we are the ones who are contributing to the increasing odds of their
extinction. 1, 2, 6, 7, 9 and 10
WHAT
IS BEING DONE & HOW CAN YOU HELP?
The first
step was, of course, to protect the Great White in their remaining ‘hotspots’
around the world by placing it on the threatened species list. South Africa was
the first country in the world to do this, back in 1991. Other countries,
including Australia, the state of California and the eastern coast of the USA,
the Maldives and the countries surrounding the Mediterranean Sea, soon
followed. The Great White Shark travels huge distances across open oceans to
other countries. Purposes for them doing this are currently unknown. One theory
is for reproductive reasons. To ensure the survival of this species, a plan
needs to be distributed and enforced globally.
With more profound monitoring from
the Fisheries, trading of body parts of the Great White Shark on the black
market has decreased. Closer monitoring of the commercial fishing industry
needs to be implemented to reduce the amount of by-catch each year and needs to
be accurately recorded. Research programs need to be further developed to find
out more about the sharks’ migration patterns, reproduction cycles, when and
where they breed and where their potential nursing grounds are.
More funding is required to continue
and further develop this vital research, and this is where you can play a very
important part!
ADOPT A GREAT
WHITE SHARK
Without your
help, extinction is looming for the Great White Shark…
Sponsor a
Great White Shark and join the Fox Shark Research Foundation (FSRF) in learning
about and protecting the sharks that live in our waters. Through the adoption
package and the research you will be supporting, you will be provided with a
window into their world through which you will discover that each shark has a
name, personality and a history.
Perhaps the
most important thing you will receive from your sponsorship is the peace of
mind that comes from knowing that you are making a real contribution to the
protection of our Great White Sharks.
A shark
adoption will mean that you are supporting vital research. By becoming an
adoptive parent, you are making a huge difference to the future of these sharks
and the marine life as a whole.
Adopt a shark
and gain an appreciation of the beauty of these magnificent predators, together
with awareness of the importance of their role in a fragile marine environment.
Sponsors receive an attractive adoption pack as a symbol of thanks to you for
supporting their (FSRF) critical work to conserve this extraordinary animal.
There are different levels of sponsorship which, depending on which level you
choose, will determine what you receive in your package. Sponsoring a shark for
yourself, or as a unique and thoughtful gift for someone special, is easy.
Simply select a shark and preferred level of sponsorship and complete the on
line sponsorship form.
WHY SPONSOR A
GREAT WHITE SHARK?
Man has long
feared sharks as the man-eater of the sea, a reputation that is highly
undeserved. The number of people killed by sharks is very small. In fact, for
every human killed by a shark, humans kill 100 million sharks worldwide.
Despite these
facts, the Great White Shark is relentlessly persecuted and their declining
numbers are fast becoming of great concern.
There is an
urgent need for researchers to find out more about this species so they can
survive into the future.
The FSRF needs
your help so researchers can continue their important work studying the sharks
that live in the Southern Ocean. Learning more about these animals and threats
they face will increase their chances of survival.
Over the past
few years, FSRF have clearly identified 200 individual sharks from their tags
and the photographic identification of their distinctive markings. Some of
these sharks, are never seen again, whilst others revisit the islands year
after year. 1
ABOUT THE
SHARKS
The sharks
offered for adoption are those who show distinctive marks, or have been star
performers on several previous expeditions dates, preferably spanning more than
one year and whom they expect to identify more reliably again in the future so
that FSRF can keep you updated on their movements.
By taking detailed photos of the first dorsal fin and the caudal fin,
researchers can closely examine indentations to identify individual sharks.
Another means
of identifying sharks is by studying pigmentation patterns on the skin in areas
between the dark upper part of the body and the pale lower part of the body,
especially around the gill slits, pelvic fins and the lower caudal fin lobe.
ADOPTION
PACKAGES
For more
information on the adoption packages please visit the FSRF website. An adoption
pamphlet is also available at their shark museum.
If you are
unable to adopt a Great White and would still like to help and contribute to
their research, please visit the Shark museum and you can personally make any
size donation.
SAFETY
RECOMMENDATIONS
Because Great
White Sharks are very inquisitive, big and powerful, and have very sharp
serrated teeth, we still need to be cautious when we play in their world. What
may seem gentle and inquisitive to them can be fatal to us. So that is why I
have included this section on safety for divers, surfers and every other beach
goer. Some safety tips are for people doing specific activities, however, the
majority is for everyone in general.
SHARKS THAT
ARE KNOWN TO BE DANGEROUS:
The following
animals have been identified in fatal unprovoked shark attacks on humans in
Australia: -
Great White
Shark (Carcharodon carcharias)
Tiger Shark (Galeocerdo
cuvier)
Bull Shark (Carcharhinus
leucas)
SHARKS THAT
HAVE BEEN CONSIDERED POTENTIALLY DANGEROUS:
Great
Hammerhead (Sphyrna mokarran)
Blue Shark (Prionace
glauca)
Mako (Isurus
oxyrinchus)
Bronze Whaler
Shark (Carcharhinus brachyurus) a.k.a. Copper shark
Grey Nurse
Shark (Carcharias taurus)
Tips For
Divers
Never Dive
alone.
Never attach
any marine organisms directly to yourself.
Avoid diving
in the vicinity of seal and sea lion rookeries or haul out sites.
Avoid diving at
times of fish spawning (especially snapper).
Be aware of
changes in your surroundings for example: marine life acting erratic or spooked
all of a sudden.
When returning
to the surface, constantly rotate and look in all directions. The majority of
attacks on SCUBA divers took place at depths of 3-6m below the surface in water
over depths of about 30-45m.4
If You See a Shark
Never provoke
any shark, no matter how small or harmless it may appear.
Never corner a
shark or cut off its path to open water.
If air supply
permitting, remain as close to or at the bottom as long as possible and wait
until the shark loses interest and leaves the area.
Leave the
water as quickly and quietly as possible (making the movement with your feet
and arms as smooth as possible).
Tips For
Surfers
Never surf
alone.
Do not surf
those locations known to be frequented by Great White Sharks.
Avoid surfing
in areas that are inhabited year-round by a population of seals and sea lions.
Avoid lengthy
periods in water with a drop-off adjacent to the surf zone.
Avoid surfing
at sunrise and sunset.
Tips For All
Beach Goers
Don’t swim
near people fishing in boats, or spear fishing.
Do not swim in
dirty or turbid water.
Avoid swimming
well offshore, or along drop-offs to deeper water.
If schooling
fish start to behave erratically or congregate in large numbers, leave the
water.
Do not swim
with pets and domestic animals.
Look carefully
before jumping into the water from a boat or wharf.
What to do if
a Shark is sighted in your area
Leave the
water as quickly and quietly as possible (making the movement with your feet
and arms as smooth as possible. The splashing could attract the shark by
increasing its curiosity).
Report shark
sightings to Fishwatch (Ph: 1800 065 522) or Police (Ph: 131 444).
What to do if you or a friend is attacked
If attacked by
a shark, try to remain calm.
Remove
yourself or the victim from the water as mentioned above, as quickly and
quietly as possible.
Once out of
the water, avoid moving the victim.
Make every
effort to control the bleeding by applying pressure on the wound or just above.
A tourniquet may be used if the bleeding can’t be controlled by a pressure
bandage. The cord or strap from surf and/or body boards are useful if there’s
nothing else at your disposal.
Do not remove
the victim’s wetsuit.
Make the
victim as comfortable as possible by laying them down and keep them warm by
covering them up.
Call 000 for
emergency service and tell them your exact location and nature of the injury.
While waiting
for the ambulance keep the victim hydrated by giving them water.
Keep
reassuring them and remain calm.1, 2, 3, 4 and 6

Additional Great White Shark Research Organisations
Andrew and
Rodney Fox’s Fox Shark Research Foundation is just one of many organisations
that are devoted to researching Great White Sharks and the survival of the
species. I have listed a few organisations and their web sites here, so you can
check them out and see what is being done around the world and what is involved
in protecting this great, poorly misunderstood creature.
Shark Research Institute (SRI) http://www.sharks.org/
The Shark
Research Institute is a multi-disciplinary non-profit 501(c)(3) scientific research
organization. It was created to sponsor and conduct research on sharks and
promote their conservation.
Founded in
1991 at Princeton, New Jersey, USA, SRI has field offices in Florida,
Pennsylvania and Texas, as well as Australia, Canada, Ecuador, Honduras, India,
Mexico, Mozambique, Seychelles, South Africa and the United Kingdom.
Its Mission is
to promote public awareness of sharks and their vital role in the marine
ecosystem.
By visiting
their web site you can adopt a shark, learn more about their history, whale
sharks and shark attacks.
CSIRO
Scientists at
CSIRO Marine and Atmospheric Research (CMAR) track Great White Shark movement
in Australian waters, using a range of tag types to learn more about these
movement patterns. To learn more about Great White shark movement patterns
visit the following web address: -
http://www.cmar.csiro.au/research/sharks/whitesharks/ozmovements.html
South African White Shark Research Institute
The White
Shark Conservation, Education and Exploration Society is an organisation that
is dedicated to the exploration and conservation of the Great White Shark and
the preservation of its environment. Visit their web site at http://www.whiteshark.co.za/ to learn
more about conservation on Great White Sharks, membership and adoption
packages.
The Conservation Council’s White Sharks Count project
Chris Ball, Marine
Programs Manager, CCSA 0408 089491
This is a
program where the public can be actively involved in reporting shark sightings.
Commercial and recreational fishers, charter boat operators and other users of
Eyre Peninsula’s marine environment are asked to report any sighting while out
on the water. Chris Ball, the Marine Programs Manager for the Conservation
Council, believes White Sharks Count will significantly increase the knowledge
of White Shark movements across the Eyre Peninsula region. Sightings can be reported a number of ways.
Go to the following web site to learn more: -
http://www.ccsa.asn.au/index.php?option=com_content&task=view&id=5542&Itemid=351
REFERENCES & More Information
1
Andrew Fox/Fox Shark Research Foundation:
Rodney
Fox Shark Museum, Moseley Square, Glenelg, South Australia 5045
2 R.
Aidan Martin/ReefQuest Centre for Shark Research:
www.elasmo-research.org/education/white_shark/overview.htm
3
John West/ASAF Taronga Zoo New South Wales:
www.zoo.nsw.gov.au/content/view.asp?id=126
Florida Museum of Natural
History:
4
International Shark Attack File (ISAF) –
www.flmnh.ufl.edu/fish/sharks/ISAF/ISAF.htm
5
“First person account on Killer Whale Vs. Great White – Predation on a white
shark (Carcharodon carcharias) by a killer whale (Orcinus orca)
and a possible case of competitive displacement” by Pyle, P; Schramm, MJ;
Keiper, C; Anderson, SD, Marine Mammal Science [Mar. Mamm. Sci.]. Vol. 15, no.
2, pp. 563-568. Apr 1999.
6
Field Guide to the Great White Shark
by R. Aidan
Martin, ReefQuest Centre for Shark Research, Special Publication No. 1, 2003
(with special contributions by Jeremy Stafford-Deitsch, Jeff Kurr, Caterina
Gennaro and Ralph S. Collier).
ISBN:
0-9732395-0-6
7 White
Shark (Carcharodon carcharias) Recovery Plan
July 2002,
Commonwealth of Australia, 2002. ISBN 0642548218
Marine
Conservation Branch, Environment Australia, GPO Box 787, Canberra, ACT 2601
www.deh.gov.au/coasts/species/sharks/greatwhite/index.html
8 2 minutes
of rare footage of a Killer Whale attacking a Great White Shark:
www.cnn.com/EARTH/9710/08/whale.vs.shark/
9
Environmental Biology of Fishes
Publisher:
Springer Netherlands, ISSN: 0378-1909 (Paper) 1573-5133 (Online)
DOI:
10.1023/A:1007639324360, Issue: Volume
58, Number 4, August 2000 (Pages: 447 – 453, Predation by White Sharks
Carcharodon carcharias (Chondrichthyes: Lamnidae) Upon Chelonians, with New
Records from the Mediterranean Sea and a First Record of the Ocean Sunfish Mola
mola (Osteichthyes: Molidae) as Stomach Contents) by Ian K. Fergusson, Leonard J.V. Compagno and Mark A. Marks.
10 CSIRO
Marine Research/Tagging Great White Sharks:
www.marine.csiro.au/research/whitesharks/index.html
11
Article on the Great White Shark being on the WWF Top Ten Extinction List:
www.sharkfoundation.com/news_archive.htm
12 www.ministers.sa.gov.au/minister.asp?mId=2&pId=6&sId=3889
13 www.themercury.news.com.au/common/story_page/0,5936,12796171%255E3462,00.html
Additional References
Marine Biology
Publisher:
Springer Berlin / Heidelberg. ISSN: 0025-3162 (Paper) 1432-1793 (Online)
DOI:
10.1007/s002270000489, Issue: Volume
138, Number 3, March 2001, Pages: 617 – 636 - The hunting strategy of white
sharks (Carcharodon carcharias) near a seal colony.
Environmental Biology of Fishes
Publisher:
Springer Netherlands. ISSN: 0378-1909 (Paper) 1573-5133 (Online)
DOI:
10.1023/A:1007520931105, Issue: Volume
56, Number 4, December 1999,
Pages: 351 –
364.
Space Utilization and Swimming Depth of White Sharks, Carcharodon
carcharias, at the South Farallon Islands, Central California
Kenneth J.
Goldman and Scot D. Anderson.
A review of the biology and status of white sharks in Australian waters
by Malcolm, H;
Bruce, BD; Stevens, JD, CSIRO, Hobart, Tas. (Australia), September 2001.
Journal of Comparative Physiology B: Biochemical,
Systemic, and Environmental Physiology
Publisher: Springer Berlin / Heidelberg. ISSN:
0174-1578 (Paper) 1432-136X (Online)
DOI: 10.1007/s003600050092, Issue: Volume 167, Number 6, August 1997, Pages: 423
– 429 - Regulation of body temperature in the white shark, Carcharodon
carcharias, Kenneth J. Goldman
Environmental Biology of Fishes
Publisher: Springer Netherlands. ISSN: 0378-1909
(Paper) 1573-5133 (Online)
DOI: 10.1023/A:1007308406137, Issue: Volume 50, Number 1, March 1997, Pages: 61 –
62 - Threatened fishes of the world: Carcharodon carcharias (Linnaeus,
1758) (Lamnidae), Leonard J.V. Compagno, Mark A. Marks and Ian K. Fergusson.
Conservation Genetics
Publisher: Springer Netherlands, ISSN: 1566-0621
(Paper) 1572-9737 (Online)
DOI: 10.1023/A:1024771215616, Issue: Volume 4, Number 4, July 2003, Pages: 415 –
425 - A streamlined, bi-organelle, multiplex PCR approach to species
identification: Application to global conservation and trade monitoring of the
great white shark, Carcharodon carcharias, by Demian D. Chapman, Debra
L. Abercrombie, Christophe J. Douady, Ellen K. Pikitch, Michael J. Stanhopen
and Mahmood S. Shivji1
Predatory behaviour of white sharks
(Carcharodon carcharias) at Seal Island, South
Africa
by R. Aidan Martin, Neil Hammerschlag, Ralph S.
Collier and Chris Fallows.
J. Mar. Biol. Ass. U.K. (2005), 85, 1121 1135
Printed
in the United Kingdom
ACKNOWLEDGEMENTS
Special
thanks to:
Andrew
Fox, Fox Shark Research Foundation (FSRF), for his support, feedback,
information, advice and valuable time
Chris
Fallows, apexpredators.com, for his contribution and use of photos. (To see
more of Chris’s magnificent photos of the Great White Shark visit www.apexpredators.com)
John West,
Australian Shark Attack File (ASAF), for the statistics and tables on shark
attacks within Australia.



“The
Fox Shark Research Foundation encourages and supports the initiative of Phil
Kemp in raising positive public awareness of the Great White Shark in the local
South Australian community. This follows our mission and philosophy that
education will always overcome fears, and aid in the protection and
conservation of this much-misunderstood animal. With more understanding and
research we can better learn to live with, change the image and reduce the
level of conflicts this species has unfortunately long attracted from a largely
irresponsible and sensationalist media and a largely misinformed and terrified
public.”
Andrew
and Rodney Fox
Fox Shark
Research Foundation

by Scoresby A. Shepherd
The
western blue groper is unique on South Australia’s reefs. It is both the
largest reef-dwelling fish, and also one of the slowest growing species,
reaching sexual maturity at 55-60 cm at 15 years of age, and a maximum length
of 1.7 m at about 70 years. For divers, it is a special treat to see a groper
swimming slowly and majestically in the water, approaching one fearlessly, and
then following with disarming curiosity.
The
groper lives in small ‘family’ groups of one male and female, together with a
few sub-adults, and occupies a home range often extending over 500 m of
coastline. During territorial disputes over boundaries, males may be seen with
jaws interlocked in a fierce combat as each struggles for supremacy. However
such events are rare, and mostly the groper swims placidly over its territory,
occasionally diving to the bottom to roll over boulders in search of small
crabs, sea-urchins or molluscs. The adults also have a remarkable bite-and-suck
behaviour, by which they can dislocate their jaw and open it widely to bite
large chunks of the algal mat, suck them into the gaping mouth cavity, and then
filter out the tiny crustaceans living in the mat. This manner of feeding is
hard work, and it is not surprising that the groper takes greedily a piece of abalone
or other shellfish offered by a passing diver.
My
interest in the groper started in the 1960s when I saw their numbers dwindle
along South Australia’s coast under the onslaught of spear fishing. These
majestic fish were easily speared and so doomed by their disarming curiosity.
In 1971 when preparing fishing controls I was able to fully protect them in the
Gulfs, and provide partial protection outside the gulfs. Protection was timely,
and seemed to arrest their decline, so that groper began to increase in
numbers. However, recent studies with James Brook and colleagues from Reef
Watch show that small groper are still captured by recreational fishers, even
though protected, and that its numbers are still almost certainly less than
they once were. This may well be because many fishers do not recognize that the
small greenish “rock cod” that they have caught is in fact a young groper. And
certainly on southern Yorke Peninsula, where adults were once common, they are
still quite rare. But what do we know of the fish’s life history?

Western Blue Groper - Photo David Muirhead
After
a spring-to-autumn reproductive season, groper larvae are believed to drift in
the sea for 3-4 weeks and perhaps up to 40 km before settling among shallow
inshore reefs of about 1 m deep, where they feed on tiny mussels and
crustaceans. As they grow they move into slightly deeper water of 2-3 m when
they are 15-20 cm long and have a dull grey-green colour matching the seaweed
in which they hide. During the next sub-adult stage lasting 10 years or more
when they are 20-60 cm long they remain in sheltered waters and become a
uniform pale green in colour. Surveys along the South Australian open coasts
show that they are in densities of 1-8 per 100 m of coastline, showing that they
are by no means an abundant species.
Sexual
maturity is at about 60 cm and occurs at about 15 years of age. They then
change colour to the more familiar blue, the females being somewhat greenish
blue compared with the deeper hue of the male. By this size they have moved to
deeper water and some migrate even further offshore to deeper reefs of 30-50 m,
where they feed on crabs, molluscs, sea-urchins, and worms.
Is
the blue groper worth protecting? Quite
apart from its majestic dignity, and friendly disposition to divers, scientists
believe that the groper plays an important key role in reef ecosystems. It is a
major prey of the spiny sea-urchin, which in large numbers can form feeding
fronts, and devastate natural reefs, leaving what are known as sea-urchin
barrens devoid of algae. Such barrens are already increasing along the east
Tasmanian coasts, likely through global warming, reducing the productivity of
the reefs by 80-90%. By ensuring that natural populations of urchin predators,
such as the groper, are present, the naturalness and productivity of coastal
reefs can be assured.
As the major
threat to groper is their continuing capture, it is surely time for us to press
for their complete protection, a matter which has been agreed to by the Fishery
Management Committee, but is still pending. In addition we must continue to
educate the public about them, as they are truly an iconic species. As such
they must have high economic value for dive tourism, and this is yet another
argument that can be used to press for their protection and for public
education.

Juvenile Western Blue Groper - Photo
David Muirhead
The Flora and Fauna of Piccaninnie Ponds and Ewens Ponds (Including Eight
Mile Creek)
by Steve Reynolds
After diving
and snorkelling in both Piccaninnie Ponds and Ewens Ponds (including Eight Mile
Creek) during the Marine Life Society’s trip to Port MacDonnell in February
2006, I wanted to list the many freshwater species of fish, plants and
invertebrates that we saw there. Since I am not an expert on the identification
of freshwater species, I enlisted the help of Mike Hammer, the Scientific
Officer for the Native Fish Australia (SA) group.
Mike got
me started by sending me an electronic copy of his report “The South East Fish
Inventory: Distribution and Conservation of Freshwater Fishes of South East
South Australia”.
Thanks to
Mike’s help, I am able to list the many species known to occur in both ponds
and Eight Mile Creek. It seems to me that I possibly saw most of these species
there. Other members of our group may possibly have sighted those species that
I didn’t see myself.
The
Piccaninnie Ponds are within the Piccaninnie Ponds Conservation Park, which is
under the control of the Department for Environment and Heritage, National
Parks and Wildlife (Mount Gambier). Permits to either dive or snorkel in the
ponds must be purchased from the DEH office at 11 Helen St, Mount Gambier (PO
Box 1046, Mt Gambier SA 5290). Contact numbers are: - telephone 8735 1171 &
8735 1177, fax 8735 1110 & 8735 1135. Diving permits are only issued to
current financial members of the Cave Divers Association of Australia who are
rated at sinkhole category.
The Ewens
Ponds are within the Ewens Ponds Conservation Park, which is also under the
control of the Department for Environment and Heritage, National Parks and
Wildlife (Mount Gambier). Permits are not required to dive or snorkel in the
ponds unless a group is larger than six divers or snorkellers. Groups of more
than six divers/snorkellers must book with the DEH office at Mount Gambier.
Fishing is not allowed in either of
the two conservation parks. Removal or damage to any plants or animals,
including fish, freshwater crayfish and yabbies, is prohibited.

Common Galaxias (Courtesy of Mike Hammer)
This first part of my list consists
of just native species of fish known to occur in the ponds and creek: -

Common Name
|
Scientific Name
|
Family
|
Conservation
Status
|
|
Common Galaxias |
Galaxias maculatus
|
Galaxiidae |
- |
|
Spotted
Galaxias |
Galaxias truttaceus
|
Galaxiiidae |
Endangered |
|
Southern
Shortfinned Eel |
Anguilla australis
|
Anguillidae |
Rare in SA |
|
River Blackfish |
Gadopsis marmoratus
|
Gadopsidae |
Protected,
Endangered |
|
Variegated
(Ewens) Pygmy Perch |
Nannoperca variegata
|
Nannopercidae |
Vulnerable
Protected, Endangered |
|
Southern Pygmy
Perch |
Nannoperca australis
|
Nannopercidae |
Protected,
Endangered |
Congolli
|
Pseudophritis urvilli
|
Bovichthidae
|
- |
Total: 7
|
|
||

Variegated Pygmy Perch (Courtesy of Mike Hammer)
As I said
in my article “Ewens Ponds” (MLSSA Newsletter, January1998, No.240), the Ewens
(or Variegated) Pygmy Perch occurs “only in Ewens Ponds and a few minor
wetlands in the lower Glenelg River system in Victoria. It is a small species
which reaches only 62mm in length”. As I said in my article “Endangered
Freshwater Species Protected in S.A.” (MLSSA Newsletter, July 1998, No.246),
“The IUCN rated the species as “vulnerable”. It chooses to live in very dense
aquatic vegetation growing in flowing water. It is threatened by: -
1.
Living in only a handful of small waters
2.
By introduced predators having been liberated into most
of these clear-water systems
3.
Its preference for what is a quite rare and vulnerable habitat
“Conservation
of the Variegated Pygmy Perch – Freshwater Fish Survey of Lower South Eastern
South Australia” by Hammer, Doube and Roberts, as the title suggests, describes
the conservation of the species and its potential threats. The report also describes
Ewens Ponds and Piccaninnie Ponds in detail and features some great maps and
diagrams.
The Ewens (or Variegated) Pygmy
Perch was listed in the IUCN Red List of threatened animals in 1997. The 1998
SA Recreational Fishing Guide listed the fish as a protected species, along
with the Southern Pygmy Perch, River Blackfish and five other freshwater
species.

Blackfish
(Courtesy of Mike Hammer)
I believe
that I saw lots of River Blackfish deep within the overhang in the third pond at
Ewens Ponds. I wedged myself as far into the overhang as I could and remained
there for quite a while, observing them in the beam of my torchlight. I was
quite entranced by them and my dive buddy, Neville Skinner began to think that
I was stuck there until I backed out of the overhang. River Blackfish are
endangered and protected in SA.
We saw several eels feeding out in
the open at the bottom of the main pond at Piccaninnie Ponds. It was a slightly
overcast morning and the eels possibly considered it to still be dawn. It seems
that they generally disappear once the sun comes up. Southern Shortfinned Eels
are rare in SA.

Southern Shortfinned Eel
(Courtesy of Mike Hammer)
Congolli, which are rare in SA, are
also known as tupong or freshwater flathead. Hillary Hauser* said in “Exploring
a Sunken Realm in Australia” (National Geographic, Vol.165, No.1, January 1984)
that they occurred in Piccaninnie Ponds where they feed on the Galaxiids. Mike
Hammer has confirmed the occurrence of Congolli in Ewens Ponds, Eight Mile
Creek and Piccaninnie Ponds.

Congolli
(Courtesy of Mike Hammer)
*Hillary Hauser is the (US) author
of several books about ‘skindiving’. She also wrote “Book of Fishes”, a
comprehensive collection of the most common fish seen by divers. The 200-page
book has over 100 colour photos.
This next part of my list covers
‘marine vagrants’ known to occur in the ponds and creek:-

Common Name
|
Scientific Name
|
Family
|
SA Fishing restrictions*
|
Bream
|
Acanthopagrus australis
|
Sparidae
|
Min. legal length & bag limit applies
|
Yellow-eye
Mullet
|
Aldrichetta forsteri
|
Mugilidae
|
Min. legal length & bag limit applies
|
Marine Goby**
|
Tasmanogobius gloveri
|
Gobiidae
|
|
Smallmouthed Hardyhead
|
Atherinosoma microstoma
|
Atherinidae
|
|
Total: 4
|
|
||
* Fishing
is not allowed in either Ewens Ponds or Piccaninnie Ponds conservation parks.
** Mike
Hammer says that he caught a Marine Goby, Tasmanogobius gloveri, at the
lower end of Eight Mile Creek.
This next part of my list covers
fish species which may possibly occur in the ponds and creek but this has not
been confirmed: -

Common
Name
|
Scientific Name
|
Family
|
Conservation Status & SA
Fishing restrictions*
|
Australian Grayling
|
Prototroctes maraena
|
Prototroctidae
|
Vulnerable
|
Short-headed
Lamprey
|
Mordacia mordax
|
Petromyzontidae?
|
Endangered
|
Pouched Lamprey
|
Geotria australis
|
Petromyzontidae
|
Endangered
|
Brown Trout
|
Salmo trutta
|
Salmonidae
|
Min. legal length applies
|
Rainbow Trout
|
Oncorhynchus mykiss
|
Salmonidae
|
Min. legal length applies
|
Total: 5
|
|
||
*Fishing
is not allowed in either Ewens Ponds or Piccaninnie Ponds conservation parks.
According
to the “Native Fish in South Australia” pamphlet (mlssa 2228), which Mike
Hammer sent to me, “Lampreys have amazing body features that help them
migrate”. I didn’t see any lampreys in Eight Mile Creek myself. I did, however,
see a school of large fish which I assumed at the time to be Australian
Grayling. I don’t believe that they could have been Yellow-eye Mullet.
According to “Australian Marine Life” by Graham Edgar, “Australian Grayling
remain in freshwater as adults but have a marine juvenile stage which lasts
until they reach about 50mm in length. This species was once very common but
has declined in numbers to only a few stable adult populations, so the species
is considered threatened”. I said in my article “Ewens Ponds” (MLSSA
Newsletter, January1998, No.240), that the summer 1996 edition of “Southern
Fisheries” magazine (Vol.4, No.4) said that Australian Grayling, Prototroctes
maraena, “has only been recorded from Ewens Ponds. It has been listed as
being close to extinction” (p.43).
The
“Freshwater Fishes of South Eastern SA data sheet” says that “Australian
Grayling have been previously recorded from the lower south east; it is either
an irregular visitor or is now locally extinct”.
Mike
Hammer referred to Australian Grayling in “The South East Fish Inventory”,
saying that they are an additional native species which have been documented in
the past, but were not captured during the inventory. Under 5.4 in his report
(Mobile species), Mike said that Grayling “does not appear to occur in SA at
the current time. Their presence in SA may be governed by a population sink
from another source such as the nearby Glenelg River subject to migration and
the health of the source population (via marine larval stage). Alternatively a
small, localized sub-population may have easily become extinct due to chance or
anthropogenic disturbance”.
Mike also said in his report that
Shortheaded Lampreys and Pouched Lampreys have been recorded in small patches
such as Ewens Ponds and Piccaninnie Ponds, but they too were not captured
during the inventory. Lampreys are endangered in SA.
This next part of my list covers
invertebrate species known to occur in the ponds and creek: -

Common
Name
|
Scientific Name
|
Family
|
Conservation Status & SA
Fishing restrictions*
|
Spiny Crayfish
|
Euastacus bispinosus (bispinosa/bispinosis/bispinosus?)
|
Parastacidae
|
Potentially threatened Bag limit applies
|
|
Burrowing
Crayfish |
Engaeus
strictifrons |
Parastacidae |
Potentially
threatened |
Freshwater crayfish
|
Geocharax species
|
Parastacidae |
Potentially
threatened |
Marron
|
Cherax tenuimanus
|
Parastacidae |
|
Yabby
|
Cherax destructor
|
|
Bag limit
applies |
Freshwater Mussel (Ridged)
|
Hyridella narracanensis
|
Mytilidae
|
Potentially
threatened |
Freshwater Mussel
|
Velesunio ambiguous?
|
|
Potentially
threatened |
Total: 7
|
|
|
|
* Fishing
is not allowed in either Ewens Ponds or Piccaninnie Ponds conservation parks.
The Spiny
Crayfish, Euastacus bispinosus (bispinosa/bispinosis/bispinosus),
is also known as the South East Freshwater Crayfish and the Glenelg River
Crayfish. It is said to be a relative of the River Murray Crayfish, Euastacus
armatus. I saw a couple of crays in Ewens Ponds, one large specimen and one
small one.
The
report titled “Observations on the ‘Mechanical Dragging’ of Eight Mile Creek,
South-east South Australia” by Mike Hammer, our own Neville Skinner and Tim
Playford (Adel. Uni.), says that “The spiny crayfish Euastacus bispinosis
has a limited distribution . . . Habitat in Eight Mile Creek represents a
significant portion of the species range in South Australia” and “The spiny
crayfish is a slow growing species unlikely to adapt well to alterations in its
habitat”.
The Spiny Crayfish (lobster), Euastacus
bispinosus (bispinosa/bispinosis/bispinosus) and the
Burrowing Crayfish, Engaeus strictifrons, are considered to have a high
conservation significance due to their limited distributions in south-eastern
Australia.
Freshwater
Crayfish, Geocharax species, are said to only have a comparatively small
home range. All freshwater crayfish are often referred to as yabbies. Marron, Cherax
tenuimanus, have been reported as occurring in Ewens Ponds even though they
are an introduced species (native to WA). They are one of the largest
freshwater crayfish in the world.
“Observations on the ‘Mechanical
Dragging’ of Eight Mile Creek, South-east South Australia” says that the Eight
Mile Creek is the only area in the state where the Freshwater Mussel, Hyridella
narracanensis, is known to occur.

A Spiny Crayfish in Eight Mile Creek (Photo by
Neville Skinner)
This next part of my list covers
more invertebrate species thought to occur in the ponds and creek: -

Common Name
|
Phylum
|
Class
|
Order
|
Other molluscs?
|
Mollusca
|
|
|
Leeches
|
Annelida
|
Hirudinea
|
|
Hydroids
|
Cnidaria
|
Hydrozoa
|
Hydroida
|
Sponges
|
Porifera
|
|
|
Shrimps
|
Arthropoda
|
Malacostraca
|
Decapoda
|
Crabs
|
Arthropoda
|
Malacostraca
|
Decapoda
|
Total: 6
|
|
|
|
My
article titled “Ewens Ponds” in our January 1998 Newsletter reported that I had
seen crabs at Ewens Ponds (in July 1997). “Discover Underwater Australia” by Neville
Coleman reports that both Ewens Ponds and Piccaninnie Ponds have crabs plus
freshwater sponges, hydroids, shrimps, terrapins and frogs.
(It is
interesting to note that the “Ewens Ponds Conservation Park Management Plan:
Amendment to Plan of Management, South East, South Australia” by the Department
of Environment and Natural Resources does not indicate the occurrence of most
of these creatures.)
With terrapins and frogs in mind,
this next part of my list covers reptile and amphibian species known to occur
in the ponds and creek: -

Common Name
|
Scientific Name
|
Family
|
|
Snake-necked tortoise or Longneck Turtle
|
Chelodina longicollis
|
Chelidae
|
|
Common eastern froglet
|
Crinia signifera
|
Myobatrachidae |
|
Ground frog
|
Geocrinia laevis
|
Myobatrachidae |
|
Eastern banjoy frog
|
Limnodynastes dumerillii
|
Myobatrachidae |
|
Spotted grass frog
|
Limnodynastes tasmaniensis
|
Myobatrachidae |
|
Southern toadlet
|
Pseudophryne semimarmorata
|
Myobatrachidae |
|
Brown tree frog
|
Litoria ewingii
|
Litoridae |
|
Bell frog
|
Litoria raniformis
|
Litoridae |
|
Total: 8
|
|
||
Chelodina
longicollis is known as both the Longneck Turtle and the Snake-necked
tortoise. Freshwater turtles are called tortoises (or terrapins), so it seems
that Snake-necked tortoise would be the correct name for them. (This matter was
discussed in my article “Turtles, Tortoises & Terrapins” in our July 1999
Newsletter (No.257).)
I saw a
couple of tortoises in Ewens Ponds/Eight Mile Creek. Like the crays that I had
seen, one was large and the other one was small.
(I was pleased to recently read in
The Advertiser (11/4/06) that the Environment Protection Authority reports that
the water quality of Lake Bonney in the south-east of SA is the best that it’s
been in 30 years and that Longneck (Snake-necked) tortoises had returned
to the lake and threatened fish species were now multiplying there.)
The book
“Biological Science – the web of life” discusses the Long-necked tortoise and
its community interrelationships.
One or
two members of our four-person team which snorkelled the length of Eight Mile
Creek discovered small leeches on themselves. Mike Hammer confirmed that there
are plenty of leeches – “small black ones that get on your lips and in between
your teeth after snorkelling around at night through swampy bits!”.
Mike says
that leeches belong to the Phylum Annelida (segmented worms) and Class
Hirudinea. “The Web of Life” book confirms this and gives other details. When
discussing the Long-necked tortoise and its community interrelationships, the
book says that “Leeches feed on tortoises without killing them; they attach
themselves to the tortoises and suck their blood”.

Longneck Turtle, Chelodina longicollis, found in the River Murray
(Courtesy of Mike Hammer)
This next part of my list covers the
common reeds and bulrush that dominate the area surrounding Ewens Ponds: -

Common Name
|
Scientific Name
|
Family
|
Common reed, bamboo reed
|
Phragmites australis
|
Poaceae (Gramineae)
|
Bulrush
|
Typha angustifolia
|
Typhaceae
|
Total: 2
|
|
|
Tea-tree
thickets consisting of Leptospermum pubescens and Scented paperbark, Melaleuca
squarrosa are scattered amongst the reeds and bulrush. These vegetation
associations (in the upper reaches of the ponds) have root systems which
stabilize the banks and prevent contamination by surface runoff.
This next part of my list covers
some of the vegetation (plant and algae species) known to occur in Ewens Ponds:
-

Common Name
|
Scientific Name
|
Family
|
#Australian lilaeopsis
|
Lilaeopsis polyantha
|
Apiaceae
(formerly Umbelliferae)
|
River buttercup
|
Ranunculus amphitrichus
|
Ranunculaceae
|
#Water ribbons
|
Triglochin procerum
|
Juncaginaceae
|
#Streaked arrowgrass
|
Triglochin striata (striatum?)
|
Juncaginaceae
|
#Shield pennywort
|
Hydrocotyle verticillata
|
Apiaceae (formerly Umbelliferae)
|
Fennel Pondweed, sago pondweed
|
Potamogeton pectinatus
|
Potamagetonaceae
|
Watercress
|
Rorippa
nasturtium-aquaticum (also called Rorippa officinalis or Nasturtium
officinale or Radicula nasturtium-aquaticum) |
Brassicaceae (Cruciferae)
|
Lesser Water parsnip
|
Berula erecta (or Sium latifolium)
|
Apiaceae (formerly Umbelliferae)
|
Spike-rush
|
Eleocharis acuta
|
Cyperaceae
|
Freshwater red alga
|
Batrachospermum species
|
Division: Rhodophyta
|
*Blue-green bacteria/alga
|
Anabaena species
|
Division: Cyanobateria
|
*Blue-green bacteria/alga
|
Oscillatoria species
|
Division: Cyanobateria
|
*Blue-green bacteria/alga
|
Lyngbya
species
|
Division: Cyanobateria
|
Moss
|
Fissidens rigidulus
|
|
Total: 14
|
|
|
# Dominant
species found in Ewens Ponds. These species range in depth from the surface to
approximately 5m. Below that level they are unable to consolidate the fine
organic matter which overlies the sands. As a consequence, blue-green bacteria
form dense mats.
*
Blue-green bacteria present below 5m in Ewens Ponds.
The
freshwater red alga, Batrachospermum species is “locally abundant” but
it is often classified as rare. It is said to be present within the small cave
(overhang) at the bottom of the third pond and also beneath the landing of the
first pond at Ewens Ponds.
The
channels between the ponds at Ewens Ponds are said to be dominated by the
watercress Rorippa nasturtium-aquaticum (also called Rorippa
officinalis or Nasturtium officinale or Radicula nasturtium-aquaticum),
the Lesser Water parsnip, Berula erecta (or Sium latifolium) and
the common spike-rush (Eleocharis acuta).
Bob
Baldock from the State Herbarium says that the lesser water parsnip Berula
erecta, is an introduced plant from western Europe, central Asia and North
America. It belongs to the family Apiaceae (formerly Umbelliferae). Bob
explained to me that the Adelaide Herbarium still uses the old Family name of
Umbelliferae. Sium latifolium may be present in SA, but there are
no reliable records.
Many of
the plants which are submerged in the ponds at Ewens Ponds occur elsewhere but
are only partly submerged in marshes. These plants that are submerged at Ewens
Ponds survive fully submerged due to water clarity. The plants are able to
obtain carbon dioxide for photosynthesis from the water and essential nutrients
are obtained by the roots from the soil.
The
Shield Pennywort, Hydrocotyle verticillata, for example, is usually
recorded as a bog species which is never submerged, but in Ewens Ponds it is
only found beneath the water surface. The moss, Fissidens rigidulus, is
usually found within the spray zone of waterfalls but it too is completely
submerged at Ewens Ponds.
According
to Hillary Hauser in her National Geographic article “Exploring a Sunken Realm
in Australia” (Vol.165, No.1, January 1984), Australian Lilaeopsis is a
relative of celery and Water ribbon (Triglochin) is found in fresh
waters across Australia. It produces an edible, potato-like tuber which
northern Aboriginals harvest. Bouquets of the River Buttercup, Ranunculus
amphitrichus, climb stalks of the Water ribbon, Triglochin procerum.
The red leaves of Ranunculus amphitrichus along the shoreline of
Piccaninnie Ponds are frosted with wisps of algae. The slightly saline aquifer
that feeds Piccaninnie Ponds seems to inhabit the spread of Ranunculus
amphitrichus, which adjusts its red pigment as needed to protect against
the sunlight drenching these crystalline waters.
“Observations
on the ‘Mechanical Dragging’ of Eight Mile Creek, South-east South Australia”
says that Eight Mile Creek has a profusion of submerged aquatic plants (such as
the pondweed Potamogeton pectinatus), many of which are normally
only found growing emerged (e.g. the Australian lilaeopsis, Lilaeopsis
polyantha, Shield pennywort, Hydrocotyle verticillata and Watercress,
Rorripa nasturtiumaquaticum). The report also says that the Water
ribbon, Triglochin procerum reaches an unusually large size in Eight
Mile Creek. It also says that “this form of habitat and mixture of species is
quite rare at the regional and state level” Aquatic plants also provide faunal
refuge (shelter) from flow and predators, as well as surfaces for invertebrates
to colonise (e.g. potential food source for fishes). Riparian vegetation is
today of limited extent along the creek, with some overhanging cover such as
grasses, emergent plants e.g. Phragmites (P.australis) and Typha
species (bulrushes), and a general mix of species that help stabilize soft
creek edges (at least in the upper reaches)”.
Hauser’s
article “Exploring a Sunken Realm in Australia” said that “The Eight Mile Creek
Swamp that once surrounded Ewens Ponds has been drained for farmland since
before the Second World War. The pond water levels, now apparently stabilized,
lie one and a half meters (five feet) below their original marks, and many of
the plant species still found at Piccaninnie Ponds have vanished from Ewens”.
Hauser’s
article also said that Galaxiids in Piccaninnie Ponds feed on algae and mosses
that build into green underwater castles. Congolli hide in the tangled cloud of
algae and lie in wait for feeding Galaxiids, which are one of their favourite
foods.
Bob Baldock
from the State Herbarium helped me out with some details about these plant
species, including comments that the Aboriginal names for Triglochin
procerum are “Narelli” and “Pol-an-go”. Bob suggested that I visit www.flora.saugov.sa.gov.au for
more details.
(The
State Herbarium maintains records of local plants, including marine species.
The herbarium probably has the largest algal collection in Australia but the
bulk of it has not been data based. This is a great pity because many marine
projects are hampered by the difficulties in accessing data on the distribution
of species from the 90,000 individual specimen sheets. A complete database
would circumvent this problem. The herbarium had sufficient funding to data
base all of the terrestrial collections but there was nothing left to database
the bulk of the algal collections. About $400,000 is needed to be able to
database the complete algal collection. Our politicians need to inject some
cash into the herbarium’s work so that our plant records may finally be
completed.)
WA
has an Internet-based record of its marine plants, the FloraBase information
system -http://www.naturebase.net/florabase.
The database provides on-line access to about 1,000 species of WA's marine
macro algae and access details of some 20,000 specimens. All of WA's marine
macro algae specimens are now housed in the Department of Conservation and Land
Management (CALM) Herbarium's algal herbarium, and about 14,000 have now been
entered on the database and added to the original 6,000 sheets at the
herbarium.
According
to the FloraBase web site: - Lilaeopsis polyantha, Ranunculus
amphitrichus and Triglochin species are all herbs. Both Lilaeopsis
polyantha and Ranunculus amphitrichus are perennials. The Australian
Lilaeopsis, Lilaeopsis polyantha is said to grow in sandy mud at lake
margins. It has purple, red or brown flowers. The River Buttercup, Ranunculus
amphitrichus has yellow flowers and is said to grow in swamps and shallow
water. Water Ribbons, Triglochin species, are “annual or perennial”.
I
couldn’t find Hydrocotyle verticillata on the FloraBase web site, so I
took Bob Baldock’s advice and visited the www.flora.saugov.sa.gov.au site
where I found some details at http://www.flora.sa.gov.au/cgi-bin/texhtml.cgi?form=speciesfacts&family=Umbelliferae&genus=Hydrocotyle
.
It seems
that pennyworts (Hydrocotyle species) are also perennial herbs
(with prostrate or ascending stems), or small annuals with erect or ascending branched
stems.
As mentioned earlier, Bob Baldock explained to me that the Adelaide Herbarium
still uses the old Family name of Umbelliferae for Hydocotyle and Lilaeopsis
species. He also helped me out with details about some of the other plants
listed above. He was able to tell me, for example, the common names, the
complete (& correct) scientific names and the Family names for them all. He
also explained that there are five other species of Potamogeton,
distinguished by the shape of their leaves. He told me that the Watercress, Rorripa
nasturtiumaquaticum is an introduced water plant and that there are four
native terrestrial species as well.
Many pest
plants, mammals and one fish species are known to occur in the ponds at Ewens
Ponds. The mammals include rats, mice, rabbits and foxes. The one pest fish
that is known to occur there is the Rainbow Trout, Salmo gairdneri,
which is an introduced species. The rabbits and foxes are also introduced
species. These introduced species have the ability to impact on the threatened
fauna found in Ewens Ponds, including native fish and crustaceans.
A fish
(trout) farm adjoins the Ewens Ponds Conservation Park to the east. It extracts
water via a channel from Pond 2 and discharges effluent via an outlet channel
into Pond 3. The discharges from the trout farm and the water quality in the
ponds is said to be monitored regularly.
According
“Exploring a Sunken Realm in Australia” by to Hillary Hauser (National
Geographic, Vol.165, No.1, January 1984), botanist Dr Neil Hallam, a professor
at Monash University spent many years studying both Ewens Ponds and Piccaninnie
Ponds. In the early 1980s Dr Hallam and graduate students from Monash
University released rhodamine, a water-tracing dye, into Pond 1, the largest of
the ponds, at Ewens Ponds to clock the rate of water exchange. It is
interesting to note that “Dyes and other substances are not allowed to be
released in the ponds whether for photography or any other purpose. They are
illegal under the Fisheries Act, 1982”.
Whilst snorkelling down Eight Mile
Creek, Neville Skinner and I sighted a ‘bluish’ fish seemingly hiding beneath
some alga at a corner of the creek. We both commented that we didn’t recognize
the species. It seemed to be bream-like but didn’t seem to be a bream. Other
possible species that come to mind are Estuary Perch, Macquaria colonorum
and Macquarie Perch, Macquaria australisca. This latter species is said
to be coloured dark bluish-grey on its dorsal (upper) surface at times. Mike
Hammer says, however, that the occurrence of Macquarie Perch in the creek is
not likely. Estuary Perch, however, are usually coloured olive-green on their
dorsal surface.

Estuary Perch, Macquaria colonorum (Courtesy of Mike Hammer)
About the time that I was completing
this article, Mike Hammer sent me lots more reference details, including
several reports on a CD. “The South East Fish Inventory: Distribution and
Conservation of Freshwater Fishes of South East South Australia” is on the CD
along with “Conservation of the Variegated Pygmy Perch – Freshwater Fish Survey
of Lower South Eastern South Australia” by Hammer, Doube and Roberts (2000), “A
Catalogue of South Australian Freshwater Fishes, including new records, range
extensions and translocations” by Hammer and Walker, Transactions of the Royal
Society of SA (2004), 128(2), 85-97 and “Observations on the ‘Mechanical
Dragging’ of Eight Mile Creek, South-east South Australia” by Mike Hammer,
Neville Skinner and T. Playford.
Southern Pygmy Perch
(Courtesy of Mike Hammer)
The CD
also includes lots of fish photos, some of which feature in this article. The
CD has been placed into our library (mlssa 8024).
Mike also
sent me a data sheet on freshwater fishes of South Eastern SA (“Freshwater
Fishes of South Eastern SA data sheet”. This has been placed in a file along
with other information used for this article. This file has also been placed
into our library (mlssa 2228).
The
“Freshwater Fishes of South Eastern SA data sheet” gives details about lampreys,
Congolli, River Blackfish, Shortfinned Eel, pygmy perch, galaxias and other
freshwater fish species. It also features many (most) of Mike Hammer’s fish
photos featured in this article.
Trevor Watts from SARFAC also sent
me a CD of Mike Hammer’s report titled “The Eastern Mount Lofty Ranges Fish
Inventory – Distribution and conservation of freshwater fishes of tributaries
to the Lower River Murray, South Australia”. This CD has also been placed into
our library (mlssa 8025).
Spotted Galaxias
(Courtesy of Mike Hammer)
This next part of my list on Page 52
covers some of the bird species known to occur in the ponds and creek: -

Common Name
|
Scientific Name
|
Pacific Black duck
|
Anas superciliosa
|
Swamp Harrier
|
Circus approximans
|
Straw-necked Ibis
|
Threskiomis spinicollis
|
Total: 3
|
|
Many
aquatic birds, however, are said to be frequent visitors to the Ewens Ponds
Conservation Park.
Many
thanks go to Mike Hammer for both his many photographs and considerable assistance
with the above details. My thanks also to Trevor Watts from SARFAC, Bob Baldock
from the State Herbarium, Christopher Deane and Neville Skinner.
REFERENCES:
“Ewens
Ponds” by Steve Reynolds, MLSSA Newsletter, January 1998 (No.240).
“More About
Ewens Ponds” by Steve Reynolds, MLSSA Newsletter, March 1998 (No.242)
“Endangered
Freshwater Species Protected in S.A.” by Steve Reynolds, MLSSA Newsletter, July
1998 (No.246).
“The
Freshwater Ponds At Port MacDonnell” by Steve Reynolds, MLSSA Newsletter, June
1999 (No.256).
“The
Flora & Fauna of Ewens Ponds” by Steve Reynolds, MLSSA Newsletter,
September 1999, No.259.
“MLSSA’s 2006 Trip To Piccaninnie Ponds,
Ewens Ponds And Eight Mile Creek - The Unofficial Report” by Steve Reynolds,
MLSSA Newsletter (2006?)
“Dredging
of Eight Mile Creek” Parts 1 & 2, by Neville Skinner, MLSSA Newsletters,
January & February 2005 (Nos.317-8).
“Eight
Mile Creek Report” by Neville Skinner, MLSSA Newsletter, January 2006 (No.328).
“Fair go
for endangered eight” by Bryan Pierce (SARDI Aquatic Sciences), Southern
Fisheries magazine, Vol.5, No.1, Autumn 1997.
“South
Australian Recreational Fishing Guide” 1998 – Freshwater, Endangered Species.
“South
Australian Recreational Fishing Guide” 2003 – The River Murray, Protected Species.
“Exploring
a Sunken Realm in Australia” by Hillary Hauser, National Geographic, Vol.165,
No.1, January 1984.
“Coastal
Fishes of South-eastern Australia” by Rudie H Kuiter, Gary Allen P/L, 2000.
“The
Marine and Freshwater Fishes of South Australia” by TD Scott, CJM Glover and RV
Southcott, Government Printer, 1980.
“Biological
Science – the web of life” by the Australian Academy of Science, 1981.
“The
South East Fish Inventory: Distribution and Conservation of Freshwater Fishes
of South East South Australia” by Michael Hammer, 2002. A CD copy of this
report has also been placed into our library (mlssa 8024). The CD also includes
lots of fish photos, some of which feature in this article.
“Conservation
of the Variegated Pygmy Perch – Freshwater Fish Survey of Lower South Eastern
South Australia” by Hammer, Doube and Roberts (2000). A CD copy of this report
has also been placed into our library (mlssa 8024).
“A
Catalogue of South Australian Freshwater Fishes, including new records, range
extensions and translocations” by Hammer and Walker, Transactions of the Royal
Society of SA (2004), 128(2), 85-97. A CD copy of this report has also been
placed into our library (mlssa 8024).
“Observations
on the ‘Mechanical Dragging’ of Eight Mile Creek, South-east South Australia”
by M.Hammer, N.Skinner and T.Playford, report to the South Eastern Water
Conservation and Drainage Board. A CD copy of this report has also been placed
into our library (mlssa 8024).
“Native
Fish in South Australia” pamphlet (mlssa 2228).
“Freshwater
Fishes of South Eastern SA data sheet” (mlssa 2228).
“The
Eastern Mount Lofty Ranges Fish Inventory – Distribution and conservation of
freshwater fishes of tributaries to the Lower River Murray, South Australia” by
Mike Hammer, September 2004. A CD copy of this report has also been placed into
our library (mlssa 8025).
“Australian
Marine Life – The Plants and Animals of Temperate Waters” by Graham Edgar,
published by Reed New Holland, Sydney, 2000, ISBN 1 876334 38 X, (mlssa 1053).
“The
Marine and Freshwater Fishes of South Australia” by Scott, Glover and
Southcott, Government Printer, 1980 (mlssa 1009).
“Turtles,
Tortoises & Terrapins” by Steve Reynolds, MLSSA Newsletter, July 1999 (No.257).
“Marine Turtles
in SA” by Steve Reynolds, MLSSA Newsletter, June 1992 (No.179).
“More
About Turtles” by Steve Reynolds, MLSSA Newsletter, July 1993 (No.191).
“Turtle
Article” by Steve Reynolds, MLSSA Newsletter, August 1995 (No.214).
“1995
Year of the Turtle”, Editor Steve Reynolds, MLSSA Newsletter, August 1995 (No.214).
“Ewen
(sic) Ponds Conservation Park Management Plan: Amendment to Plan of Management,
South East, South Australia” by the Department of Environment and Natural
Resources. South-East Region, Natural Resources Group, ISBN or ISSN:
0730858219.
‘The
Biology of Ewens Ponds and Piccaninnie Ponds, South Australia’ by Dr. Neill
Hallam Senior Lecturer in Botany Monash Uni. February 1985 in Habitat Vol.13 No
1.
The web
site for Native Fish Australia (SA) – www.nativefishsa.asn.au
.
For more
details about freshwater turtles visit http://www.anbg.gov.au/cpbr/WfHC/Chelidae/index.html
.
For more
details about crayfish visit the following crayfish web sites (& web
pages): -
http://www.fish.wa.gov.au/docs/pub/IdCrayfish/IdCrayfishPage01.php?0304
http://www.crayfishworld.com/pictureindex.htm
(Crayfish Photo Index)
http://www.crayfishworld.com/geocharax.htm
(Geocharax species)
http://www.crayfishworld.com/spinya2.htm
(for more details about the Spiny Crayfish, Euastacus bispinosus (bispinosa/bispinosis/bispinosus)
)
by Gerard Carmody
from the
Australian New Guinea Fishes Association (ANGFA) travelled to Port MacDonnell
to visit the magnificent wetlands of the south-eastern corner of South
Australia. We came specifically to dive the uniquely spectacular and complex
groundwater dependent ecosystem of Ewens Ponds Conservation Park (EPCP). Like
the many thousands of visitors before us, the plan was to take in the
experience of the crystal clear water and abundant aquatic fauna and flora.
Unfortunately this trip was very different to past experience and expectations
as we saw the alarming deterioration of the Ewens Ponds wetland and out-flowing
Eight Mile Creek. The most noticeable change was the widespread infestation and
impact of an aggressive new form of blue-green algae throughout the ponds and
creek. Of major concern is the degradation of critical habitat for the
vulnerable Ewens Pygmy Perch (Nannoperca variegata).
On our
return from Ewens Ponds I was motivated to raise awareness of its rapid
decline, including the impact on aquatic fauna and flora. As a part of this, I undertook
research to become better informed about the issue and to alert and encourage
all responsible authorities and interested parties to take immediate action.
Is
History Repeating Itself?


Above: The Ewens Ponds Creek channel linking Pond 2 to Pond 3 – Top:
October 2002 – Bottom January 2006 –
Photographs: Neville Skinner

Above: “Welcome to Ewens Ponds” – Department of Environment and Heritage
signage – Photograph: Kath Moores
In the late
1970s to early 1980s a dieback phenomenon was reported in Ewens Ponds. This
eventually lead to its closure to divers (Lewis, Stace 1980). By the late
1980s, however Ewens had mostly recovered (although some dieback continued for
a number of years in the area of the fall-out zone from the water flowing from
the cave in pond 3, Lipson 1989). The cause of this dieback remained a puzzle
then as it does now. Are we seeing the return of this problem in 2006 or
something different?
The first
indication of the recent blue-green algae infestation in Ewens Ponds was
reported by a local Dive shop in the summer of 2004/05. Perhaps not
surprisingly, the mass outbreak of blue-algae occurred in the following summer
of 2005/06 after another year of drought.
The Change
In
previous regular visits to Ewens Ponds, aquatic vegetation such as water ribbon
(Triglochin procerum) was commonly present at depths ranging from 1 to 6
metres (Hallam 1985). Beyond this depth, filamentous green algae and other
benign blue-green algae species were present. This new infestation of
blue-green algae is out-competing the most vigorous of these aquatic plants at
depth as shallow as two metres leading to extensive die-back of aquatic plants
throughout the system. Only in sections of the Eight Mile Creek, where water
flow is significant does aquatic plant growth of species such as watercress (Rorippa
nasturtium aquaticum), shield pennywort (Hydrocotyle verticillata)
and river buttercup (Ranunculus amphitrichus) appear normal. Eight Mile
Creek, however is far from free of this new infestation.


Above: Ewens Ponds – Top: Pond 2 October 2002 – Bottom: Pond 2 January 2006
– Photographs: Neville Skinner
The
luxuriant thick mats of filamentous green algae that covered the sloping banks
of the three ponds from 5M depth have entirely disappeared and replaced with
the ubiquitous blue-green algae. The visual “sand boils” feeding water into the
bottom of the ponds 1 and 2 from the shallow-water aquifers have also
significantly declined. These changes coincide with a drastic reduction in the
previously plentiful populations of Southern Pygmy Perch (Nannoperca
australis) which would normally be seen congregating in large numbers near
these “sand boils”. Common Jollytail (Galaxias maculatus), Congolli (Pseudaphritis
urvilli), freshwater crayfish (Euastacus bispinosus), shrimp (Paratya)
and numerous other species have also declined. The exceptions are River
Blackfish (Gadopsis marmoratus) and Black Bream (Acanthopagrus
butcheri).

Above: Southern Pygmy Perch (Nannoperca australis) schooling at the bottom
of Ewens Ponds prior to the blue-green algae bloom.
Photograph: Rudie Kuiter

Above: The vulnerable Ewens Pygmy Perch (Nannoperca variegata).
Photograph: Rudie Kuiter
Of most concern
is the degradation of critical habitat for Ewens Pygmy Perch (Nannoperca
variegata), of which only a few were observed in the creek sections and a
small population congregating under the platform in pond 3. Ewens or Variegated
Pygmy Perch are listed as vulnerable by the International Union for the
Conservation of Nature (IUCN). Ewens Ponds, Eight Mile Creek, connecting
Spencer’s Pond and adjacent Stratman’s Pond are critical habitats for this
species (Hammer, Doube and Roberts 2000). The loss of Ewens Pygmy Perch from
these locations would surely place them on the endangered red list.
In July
2006 a further visit was made with Neville Skinner from the Marine Life Society
of South Australia (MLSSA) and our observations are that the blue-green algae
have spread further within the three major ponds. The extent of the problem is
clearly evident from the entry platform to pond 1 with rafts of blue-green
algae floating on the surface. It is clearly plain to the observer that major
changes have occurred which are having a very significant impact on both animal
and aquatic plant life. It is essential that the impact be quantified in terms
of important measures such as species richness, evenness and individual species
number by a follow-up survey of similar detail to the research by Hammer, Doube
and Roberts (2000).

Above: The Ewens Ponds Creek channel linking Pond 2 to Pond 3
Top: October 2002 – Bottom January 2006 – Photographs: Neville Skinner
Water: Precious Resource or Free Gift?
What makes
Ewens Ponds such a wonderfully rich and unique aquatic ecosystem are the same
things that result in the adjacent farmland being extremely attractive for
intensive agricultural use and ultimately compete with the ecosystem for these
resources (Hallam 1985, Skewes 2006). The plentiful clear and constant water
flow from the unconfined Mount Gambier aquifer and rich seam of peat soils
provide an excellent nutrient source for aquatic plants, allowing for
exceptional plant growth in the ponds and creek. So productive is this aquatic
ecosystem that certain species of plants are able to flower underwater and
oxygen is regularly observed fizzing from the tips of submerged
photosynthesising plants. The long history of flooding and interconnection with
other wetlands in the area, and Eight Mile Creek linkage with the ocean has
resulted in a diverse and rich aquatic fauna and flora (Hammer 2002).

Above: Centre Pivot Irrigators at work – Port MacDonnell
Photograph: Gerard Carmody

Above: Ewens Ponds, Pond 1 pre blue-green algae bloom
Photograph: Rudie Kuiter
Yet
despite the very special significance of this spring fed ecosystem, very little
is known about the dominant hydrogeology, whether it is fed from a deep or
shallow aquifer or the direction from which the water is flowing. Mostly, the
current information is anecdotal and from recreational SCUBA divers. Being part
of the greater Mt Gambier unconfined aquifer also makes these groundwater
dependent ecosystems extremely vulnerable to pollution from agricultural
run-off and waste disposal in sinkholes (Hammer et al 2000). The threat posed
by agricultural run-off is very real as the soil depth is relatively shallow
near EPCP.

Above: Matts of blue-green algae near jetty of Pond 1 July 2006.
Photograph: Gerard Carmody
The
majority of the farmland surrounding EPCP is utilised for intensive dairy
production which relies on an extensive artificial drainage network (post WW2
soldier settlement) to reduce water logging. In the past decade, the intensive
use of Centre Pivot Irrigation to exploit the shallow groundwater aquifer
resources and heavy fertilisation have made this area the most modern and
productive dairy country in Australia. The groundwater resources have been
utilised to maximise farm production all year round, effectively
drought-proofing them. Coinciding with this increase in shallow aquifer
extraction, the South East has experienced successive years of drought and the
rate of water recharge back into the aquifer has fallen far below the amount
extracted for agriculture (ref SENRM). The greater Mt Gambier unconfined
aquifer has significantly fallen in level and as a result the hydraulic
pressure pushing water through the aquifer is also believed to have declined.
Only since June 2006 has it become mandatory for all farm bores to be metered.
Up until then, the volume of water extraction was effectively uncapped. Recent
measurements of water flow at the mouth of Eight Mile Creek are 30% below
levels found in the 1970s (ref DEH), however it is unknown whether flow has
reduced from the three ponds or from Eight Mile Creek. In addition to
groundwater extraction, water is now drawn directly from the Ewens Ponds system
(Eight Mile Creek) for irrigation and partly returned via a complex network of
drains from the adjacent farmland. Since 1978, a trout farm has been given
license to draw water from pond 2 and discharge below pond 3 into Eight Mile
Creek. As a first and welcome step, an investigation will be carried out in
late 2006 by a joint working group, lead by the Department of Environment and
Heritage to measure water flow from pond 3 and determine the origin of the
change. The outcome of this study will help shape the future direction of
further investigations into this problem.

Above: “The dredge” chained to tractors either side of Eight Mile Creek and
dragged along, taking flora and fauna with it.
Below: Permanently damaged creek bed.
Photographs: Gerard Carmody

The Algal Bloom
Although the
aquatic chemical processes involved in blue-green algae blooms are complex,
they are known to be caused by reduced water flow and water stratification in
eutrophic (nutrient rich) systems, such as the Murray River and Gippsland Lakes
experience (Stevens 2006). Blue-green algae are able to fix their own nitrogen
in freshwater, therefore nitrogen is not considered a limiting nutrient in
Ewens Ponds. Small increases in nutrients such as phosphorus are known to
trigger outbreaks in freshwater (Stevens 2006). In times of normally high water
flow and limited nutrient input, Ewens Ponds effectively behaves as an
oligotrophic (nutrient poor) system. The present blue-green algae outbreak
points toward a significant change in water flow or nutrient input (via farm
fertilisation and run-off) and a thorough investigation of water flow and
program of water analysis against a similar control system is urgently needed.
Potential
sources of nutrient input into Ewens Ponds can be found in the adjacent
farmland. High strength liquid NPK and trace nutrient fertilisers are pumped
through giant centre pivot irrigation
systems by a process of “fertigation” (see appendix for chemical breakdown).
Optimum pasture growth is achieved with highly targeted fertiliser application,
intensive irrigation and well drained soils (Skewes 2006).
Soil Drainage and Dredging of Eight Mile Creek
Responsibility
for the management of Eight Mile Creek is with the South East Water
Conservation and Drainage Board (SEWCDB). The SEWCDB is comprised of the
landholders adjacent to EPCP and a few government representatives. As
irrigation has increased close to EPCP, drainage of water logged soils has
become more critical to optimise pasture growth. This is a challenging task as
it was once part of a major wetland system. Dredging Eight Mile Creek is viewed
by SEWCDB as a viable way to enhance soil drainage of the adjacent farmland.
Dredging in theory supposedly reduces the resistance to water flow in the creek
which then lowers the water levels in the three ponds and surrounding water
table. Increasing irrigation and fertigation adjacent to EPCP, however, may
lead to an increase in nutrient load to Ewens Ponds via run-off through the
shallow soil into aquifers or overground. If this were to occur, it may also
lead to a cycle of increased aquatic plant growth in Eight Mile Creek and in
turn greater call for dredging.
The
SEWCDB’s continued desire to dredge aquatic plants from Eight Mile Creek and
claims that Ewens Ponds would benefit, need to be seriously questioned. It is
difficult to see how dredging and dropping the water level further would
improve water flow through Ewens Ponds given the hydraulic pressure of the
aquifers feeding the ponds has probably declined. When snorkelling Eight Mile
Creek to the mouth during our recent visits, at no stage did we observe any
creek blockages caused by aquatic plant build up. The creek was free flowing
with at least a metre of clear water depth on average. Past dredging practices
have caused great destruction to habitat and loss of aquatic life (Skinner,
Hammer and Playford, 2004). Eight Mile Creek is especially important for
recruitment of fish back into the ponds if and when they recover. Any further
request for dredging should be scientifically evaluated before going ahead as
it is clearly opposite to the interests of the sustainability of EPCP.

Above: Ewens Ponds March 2006 (Pond 3) – Common Jollytail (Galaxias
maculatus) swimming amongst the blue-green algae afflicted ribbongrass
(Vallisneria americana).
By July 2006 the same aquatic vegetation had been killed off.
Photograph: Gerard Carmody
Recommendations
Urgent
and well coordinated intervention is needed to save Ewens Ponds. Specifically,
the Federal Department of Environment and Heritage (DEH) and South Australian
Department of Environment and Heritage (DEAH) must carry out their
responsibilities as custodians, according to the EPCP Management Plan (1999)
and ensure that all actions are taken to determine and implement remedial
solutions. This needs to be an absolute priority for DEH and DEAH. The South
East Natural Resource Management Board (SENRMB) must take into consideration
when developing the Natural Resources Management Plan, the requirements of
groundwater dependent ecosystems such as EPCP in groundwater allocation.
Despite
some very basic and non-specific chemical and water flow monitoring at the
mouth of eight Mile Creek, the breadth and extent of this analysis and
knowledge of the aquifers feeding the Ewens Ponds system remains inadequate
(see appendix for representative analysis). This must be rectified. The South
Australian Environment Protection Agency and other relevant working groups must
be given a key project management role to comprehensively analyse and
understand this ecosystem, including the key hydrological and chemical
processes. There must also be specific and ongoing funding and political will
to do this. Current resources allocated to this problem are grossly
insufficient. These agencies of talented scientists and professionals are capable
if given the commitment of time and resources, to develop and implement an
appropriate plan.
The water
resources of south eastern Australia are a finite and precious resource. More
precise and selective irrigation practices need to be implemented and farms
operated within a water budget. If agrochemical run-off is also found to be a
key contributor to Ewens Ponds decline, then a cap on fertiliser usage, similar
to current European farm legislation, must be adopted. Farms need to work
within tighter water and fertiliser budgets.
Unfortunately,
the Ewens Ponds Conservation Park excludes the important downstream section of
Eight Mile Creek, which has long been under the control and management of the
SEWCDB. The Ewens Ponds ecosystem and connecting Eight Mile Creek are of
exceptional ecological significance. Continued reference of Eight Mile Creek as
a “drain” is archaic and completely unacceptable. Steps need to be taken in
bringing Eight Mile Creek back into the Ewens Pond Conservation Park and move
to National Park status.
The
creation of greater buffer or riparian zones adjacent to EPCP is needed. The
current boundaries allow cattle to walk within meters of the ponds, increasing
the likelihood of agricultural run-off. The DEAH must also give consideration to
acquisition of available land for buffer zone and inclusion of Eight Mile Creek
as part of the Ewens Ponds Conservation Park as outlined in the 1999 Ewens
Ponds Conservation Management Plan.
A Call to Action
I urge
everyone who is interested in the preservation of Ewens Ponds to write, phone
or e-mail the following government representatives to express your concern.
Ewens Ponds will not survive if we remain indifferent. Immediate action is
needed and the highest priority given to this groundwater dependent ecosystem,
especially in the allocation of groundwater (i.e. the SENRMB Water Allocation
Plan).
Hugo Hopton
General Manager
South East NRM Board
PO Box 30, Mt GAMBIER, SA 5290
Ph: (08) 8724 6000
Email: nrm_admin@senrm.sa.gov.au
Ross Anderson,
District Ranger – Lower South East
National Parks and Wildlife
Department of Environment and Heritage
11 Helen Street, PO Box 1046,
Mt Gambier SA 5290,
Ph: (08) 8735 1174
Email: anderson.ross@saugov.sa.gov.au
Hon. Gail Gago
Minister for Environment and Conservation
Parliament House
Adelaide, SA 5000
Ph: (08) 8237 9100
Email: gago.office@parliament.sa.gov.au
Senator Ian Campbell
Minister for the Environment and Heritage
Parliament House, Canberra, ACT 2600
Tel: (02) 6277 7640
Fax: (02) 6273 6101
Email: senator.ian.campbell@aph.gov.au
References:
Ewens Ponds Conservation Park Management Plan, Feb 1999,
DEHAA
Hallam, N. Feb 1985, Habitat Vol 13, #1, “The Biology of
Ewens and Piccaninnie Ponds, South Australia”.
Hammer, M., Skinner, N. and Playford, T. 2004
“Observation on the Mechanical Dragging of Eight Mile Creek, South-East South
Australia”.
Hammer, M. Sept 2002, “The South East Fish Inventory”.
Hammer, M., Doube, J. and Roberts, M. Nov 2000,
“Conservation of the Variegated Pygmy Perch”, Freshwater Fish Survey of Lower
South Eastern South Australia.
Lewis, I. and Stace, P. 1980. “Cave Diving in Australia”.
Pg 60-61
Lipson, R. Dec 1989. Sports Diver, “Mount Gambier – Part
2”
Kuiter, R. H. Sept 2003,”Fishes of Sahul”, Journal of the
Australian New Guinea Fishes Association, Vol 17, No. 3 pg 953-959 “Discovering
Ewens Pygmy Perch”
Personal Communications: EPA VIC – A Stevens, MLSSA – N
Skinner, NFA – M Hammer, R Kuiter. K Smales (layout and typesetting).
Skewes, M., Treloar, N, Bailey, G. 2006. “Irrigation
Innovations in the South East of South Australia”.
Appendix:
Chemical Analysis
The
following chemical analysis is courtesy of DEH, Hammer, Doube and Roberts (2000)
and Monash University Water Studies Centre.
 Along
Eight Mile Creek one can find empty and full one-tonne boxes of “fertigation”
fertiliser by the side of the road. These are stored in the open paddock and
used in the Centre Pivot Irrigation process. The particular product used by one
farm is manufactured by the South Australian company Spraygro Liquid
Fertilisers. The product used is called BASE™ 15 - 18 - 20 +, a Nitrogen,
Phosphorus, Potassium and trace metal, high strength fertiliser (information
freely available from the product data sheet and msds listed on the Spraygro
Website). This product is a typical fertigation product and contains very high
concentration of algal growth limiting nutrients, in particular phosphorus in
the readily accessible polyphosphate form.
Along
Eight Mile Creek one can find empty and full one-tonne boxes of “fertigation”
fertiliser by the side of the road. These are stored in the open paddock and
used in the Centre Pivot Irrigation process. The particular product used by one
farm is manufactured by the South Australian company Spraygro Liquid
Fertilisers. The product used is called BASE™ 15 - 18 - 20 +, a Nitrogen,
Phosphorus, Potassium and trace metal, high strength fertiliser (information
freely available from the product data sheet and msds listed on the Spraygro
Website). This product is a typical fertigation product and contains very high
concentration of algal growth limiting nutrients, in particular phosphorus in
the readily accessible polyphosphate form.
Based on Eight Mile Creek flow rate
of 2300 L/second (Lewis 1980), a measurement of 0.01mg/L Total phosphorus
(analysis by Monash University Water Studies Centre - April 2006) correlates to
2Kg per day of total phosphorus reaching the mouth of eight Mile Creek. This is
an approximate calculation and does not take into account 2006 reduced flow
rate or phosphorus uptake by aquatic plants along the journey. Therefore a one
tonne box of BASE™ 15 - 18 - 20 + fertiliser (15%w/v Nitrogen, 18% Phosphorus,
20% Potassium, 0.4% S, 0.02% Mg, 0.1% Fe, 0.08% Mn, 0.1% Zn, 0.03% B, 0.04% Cu,
0.0017% Mo) is equivalent to 90 days phosphorus loading to Ewens Ponds.
Therefore if even a small fraction of the fertiliser applied in the Ewens Ponds
catchment made its way into the shallow aquifer or drainage channels feeding
Ewens Ponds, then it is plausible that this may be contributing significantly
to the blue-green algae problem.
Changing Rainfall:
The ten
year history of rainfall in South Australia. The Mount Gambier region
(encompassing Ewens Ponds) has received below average rainfall. The rainfall of
the catchment just to the north is very much below average during this past
decade. See chart below. Courtesy of Australian Bureau of Meteorology.
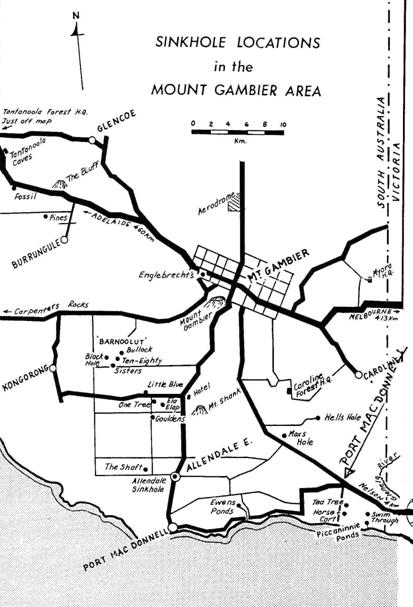

Patagonian Tooth-fish – why all the fuss?
by Evan John
Much has been
made of the poaching of the Patagonian tooth-fish in Australian southern
oceans, and on several recent occasions, newspapers have highlighted chases by
Australian naval vessels in the pursuit and apprehension of rogue fishermen
from other countries who illegally take these fish from Australian waters.
Martin Collins, Mark
Belchier and Inigo Everson of the British Antarctic Survey in
Cambridge, UK, in the June edition of Biologist, pose the question – why all
the fuss?
(Biologist, Vol
50 No 3. June 2003)
Tooth-fish, so
named because of the sharp teeth on their upper jaw, are quite large, predatory
and scavenging fish with tasty flesh, found in deep water in the Southern Ocean,
and sold commercially as Chilean seabass.

Fig 1 Patagonian
tooth-fish
(Dissostichus eleginoides)
There are two
species of tooth-fish: Dissostichus mawsoni, and D. eleginoides.
Both species belong to the family Nototheniidae, (southern cod), which
are endemic to the southern hemisphere, and are the dominant Antarctic fish
groups.
The two species
overlap in their distribution, although the former, commonly called the
Antarctic tooth-fish, is found at higher latitudes around Antarctica, whereas
the latter, the Patagonian tooth-fish,
normally occurs further north. Both species can reach sizes of over 2 metres,
and can weigh more than 100 kilograms.
Patagonian
tooth-fish are found within a broad depth range. Although little is known of
the larval and juvenile phases of their life cycles, data from the South
Georgia region suggests that juveniles generally occur in shallower water and
mean size increases with depth to a maximum of about 2,500 m. It is believed
they spawn in deep water between 500-1000m in the austral winter. Females
produce between 50,000 and 500,000 pelagic eggs about 4 - 5mm in diameter, that
hatch into small pelagic larvae. How long they remain as such is unknown, but
by the time they reach 30cm in length, they have reverted to a demersal or
bottom-associated habit. Growth rings from scales and otoliths have been used
to suggest that tooth-fish grow reasonably quickly in the first ten years
compared to other deep-sea fish, and may reach up to one metre in length, as
they gradually migrate to depths of 750-1500m where they are most abundant.
They seem to mature after this initial rapid growth, with a subsequent slowing
down of the growth rate in latter years. This may be associated with a reduced
food supply in the deeper sea, or with an annual energy investment in
reproduction. Scale and otolith analysis also suggests that females grow faster
and reach greater sizes than males, and that both sexes may reach 50 years of
age.
Fig 2. Distribution of Dissostichus eleginoides. The heavy
line indicates the area under the jurisdiction of CCALMR
This growth
rate-depth differential also suggests variations in the nature of the food
supply. Juvenile tooth-fish are visual predators, using their sharp teeth to
catch fish, crustaceans and cephalopods, with an occasional supplement of
krill. As they get older and move deeper, they most likely depend more on
olfactory senses and mechanoreceptors, found in their pronounced lateral line.
At maximum depths the larger animals probably also feed on carrion, dead fish
and squid that fall to the ocean floor, in addition to benthic fish and decapod
crustaceans normally found there. These latter animals seemingly have few
predators at that depth, although juvenile tooth-fish have been found in the
stomach contents of penguins, sea lions, sperm whales and elephant seals.
Data from tagging
suggests that there is little lateral geographical migration of larger
tooth-fish between different locations within the ocean basins, which seemingly
present a physical barrier to gene flow between different populations. Studies
have also shown that there is a genetic break between Southern Ocean
populations of Patagonian toth-fish and those of the South American plateau.
Furthermore, there are genetic differences between the isolated populations
around the sub-Antarctic islands.
D. eleginoides, the Patagonian
tooth-fish, is the target of a major and valuable long-line fishing industry,
and this leads to questions associated with sustainability and the impact on
the Southern Ocean’s ecology. With the Patagonian tooth-fish fetching in excess
of US$10 a kilogram at first point of sale, doubling before it reaches the
customer in Japan and America where it is extremely popular, it is essential to
monitor the effects of the rapid expansion, and hence potential for
over-exploitation of these Antarctic stocks since the early 1990’s.
In the late
1970’s Russian trawlers caught mainly smaller tooth-fish as a by-catch in
waters less than 200 m deep around the South Georgia Islands. It was not until
long-line fishing began in Chilean waters in the late 1980’s, targeting larger
tooth-fish, that the industry spread to the Patagonian shelf and then to the
sub-Antarctic Islands, South Georgia, Kerguelen, Heard and Macquarie.
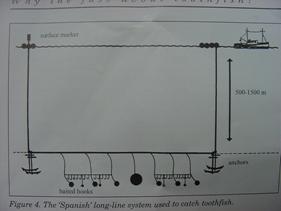
Fig 3 The “Spanish” long-line system
used to catch tooth-fish.
In the 1990’s
with market values becoming increasingly higher, long-line fishing operations
began in deeper waters (700-1500m). FAO reportings (legal) of tooth-fish landings
increased from less than 5,000 tonnes in 1984 to 40,000 tonnes in 1994. In
long-line fishing, vessels deploy lines with up to 10,000 barbed baited hooks
over the stern. The weighted lines sink to the sea floor, each end of the line
being marked with a surface buoy, where they are left for 24-48 hours before
being hauled in, with the catch being removed from the hooks as the lines are
recovered. The hooks are baited with squid or fish that attract the larger
tooth-fish, more abundant at these depths.
Concern
at the increase in illegal catches, which undoubtedly had an effect on
tooth-fish stocks as well as sea birds (see later), the Commission for the
Conservation of Antarctic Living Marine Resources (CCAMLR) set total allowable
catches (TAC’s), (admittedly
conservative, because of the uncertainty in available data about the
tooth-fish) in the mid 1990’s. In addition, genuine management (eg chasing
poachers!) within 200 mile zones around Antarctic islands by the UK, Australia,
France, and South Africa, in support of CCAMLR, producing high profile arrests
and large fines, have contributed to substantial reduction in the levels of
illegal, unregulated and unreported catches. (IUU’s). CCAMLR in 2001 also
introduced a catch documentation scheme (CDS) in which legally taken tooth-fish
command a considerably higher price than uncertified catches. IUU will always
remain a problem, but data from South Georgia indicating that TAC’s are
remaining broadly the same over the history of the fishery there, gives early
indications that there may be sustainability if current procedures are adhered
to. The Patagonian tooth-fish industry has contributed much to an understanding
of D. eleginoides biology.
What ecological
impacts are being made by the tooth-fish industry?
Long-line fishing
is far more selective than trawling, for it normally catches only scavenging
fish, and does little damage to the sea bed in comparison. There is a small associated catch of
grenadiers, skates and deepwater hake, but of more concern was the coinciding
mortality of sea birds, particularly albatrosses. In the 1970’s and 1980’s this
was attributed to the long-line fishing of tuna, and studies showed that some
albatross populations were in decline. When baited hooks pass out from the
stern of long-lining ships, albatrosses and petrels are caught and drowned
whilst trying to take the bait from the hooks.
CCAMLR introduced
measures to reduce this incidence; restriction of the setting of long-lines to
the hours of darkness, and closing the fisheries during migratory seasons when
the birds are most vulnerable. These procedures seem to have been effective,
for data collected around South Georgia indicates that sea bird mortality fell
from an estimated 3,000 during the 1992-93 season, to just 30 in the 2000-2001
season.
Unfortunately,
implementation of these measures is difficult to enforce outside the Economic
Exclusion Zones and CCAMLR’s jurisdiction. It is the so-called “pirates” that
we read about in the newspapers, operating without any controlling measures and
selling their catch illegally that cause problems. Preventing these activities
may well be absolutely necessary if the future of the Patagonian tooth-fish
industry is to become a sustainable venture.
In summary, then,
Patagonian tooth-fish are long-lived fish that develop very rapidly in their
first ten years of life, and hence may be less susceptible to overfishing than
other deep sea fish, provided that a sufficient juvenile population is
maintained in order to reach maturity and spawn. The Catch Documentation Scheme
initiated by CCAMLR has provided a framework whereby fish can be traced from
their point of capture to the shelves of the supermarket.
With the by-catch
problem of sea birds having been addressed, resolute management of illegal
fishing by countries involved in the sub-Antarctic islands, and consumers being
careful about buying fish from a certified source, there seems to be a future
for both the Patagonian tooth-fish and the fishery!
MARINE LIFE SOCIETY OF SOUTH
AUSTRALIA Inc.
2006 - 2007 COMMITTEE
PRESIDENT :
Philip Hall 82704463
SECRETARY :
Neville Skinner 82964142
TREASURER :
Phil McPeake 83841156
COMMITTEE : David
Muirhead
MEMBERS :
Chris Hall
2006 - 2007
OFFICERS
EDITOR :
Philip Hall
CCSA :
Scoresby Shepherd
:
Robert Browne
SDF :
Neville Skinner
:
Steve Reynolds
REEFWATCH :
Kevin Smith
LIBRARY : Steve
Reynolds
PHOTO INDEX : Steve Reynolds
SOCIAL : As
Needed
WEBMASTER : Danny Gibbins
AUDITOR : Phill
John
Meetings of the
Society
General Meetings of the Society
are held on the 3rd Wednesday of each month
(except December) at 7.30 pm sharp, at the:-
Conservation Council
120 Wakefield Street
Adelaide
South Australia
Parking is available
on the Tyre Depot forecourt
on the western side of the
Conservation Centre.
Produced by the
Marine Life Society
of South Australia Inc.
mlssa
http://www.mlssa.asn.au
Email marinelifesa@chariot.net.au



