Marine life Society
of South Australia Inc
2007 Journal
Number 17
December 2007
THE MARINE LIFE SOCIETY OF SOUTH AUSTRALIA Inc.
Are
you interested in any aspect of marine life? Do you want to learn more about the
underwater world? Are you concerned about pollution of our oceans and
destruction of reefs and seagrass beds? If so MLSSA is for you.
Our
motto is “--- understanding, enjoying and caring for our oceans ---”. These few
words summarise our aims. Members seek to understand our ocean, derive
enjoyment from observations of marine life and are committed to protection of
the marine environment.
Become
a Society member and enjoy contact with others with similar interests. Our
members include divers, marine aquarists and naturalists.
Our
activities include:-
-Studying
our local marine environment
-Community Education
-Underwater
photography
Established in 1976, MLSSA holds
monthly meetings and occasional field trips. We produce various informative and
educational publications including a monthly Newsletter, an Annual Journal and
a beautifully illustrated Calendar showing only South Australian marine life.
Our library is a source of helpful information for marine enthusiasts.
Through
our affiliation with other organisations (eg Conservation Council of SA and the Scuba Divers
Federation of SA) we are kept up to date with relevant issues of interest.
MLSSA also has close ties with appropriate Government organisations, e.g.
various museums, universities and libraries.
Everyone
is welcome to attend our General Meetings which are held on the third Wednesday
of every month (except January and December). As
our usual meeting place has been sold please access our website at : www.mlssa.asn.au to find out where and when the next
meeting will take place.
We begin with a guest speaker. After a short break there is the general
business meeting and this may be followed by a slide show if time permits. The
atmosphere is friendly and informal.
We
welcome new members. We have subscription levels for students, individuals,
families and organisations. We invite you to complete the membership
subscription form on our website at:- http://www.mlssa.asn.au
Or you may
wish to contact the Society for a form, or to complete the one on Page 40 of this Journal (or a photocopy) and send it with your
payment to MLSSA.
The postal
address of the Society is at present:-
MLSSA
Inc.
120 Wakefield
Street,
ADELAIDE 5000.
OUR LOGO
The MLSSA logo
on the front page features a Leafy Seadragon which is unique to southern
Australian waters. The Leafy was South Australia’s first totally protected fish and is
the State marine emblem. Its beauty surpasses that of any creature found in
tropical waters and, once seen by divers, is amongst the most remembered of
their diving experiences.
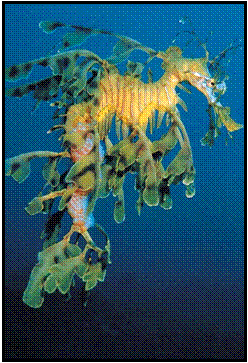
Male
Leafy Seadragon carrying eggs
Photograph
courtesy of MLSSA member David Muirhead.
CONTENTS
Cuttlefish Attacks On Divers (Steve
Reynolds)
Invasive
Marine Species: A Critical Assessment Of Global
Legislation Concerning Prevention And Control With A Particular Focus On
Ballast Water Management. (Morgan
Muirhead)
Early Settlement at Glenelg (Brian J.
Brock)
The Flora & Fauna Of Piccaninnie
Ponds And Ewens Ponds (Including Eight Mile Creek) - Part 2 (Steve Reynolds)
EDITORIAL
This year we have a variety of articles from either MLSSA members or by a
member of their family. The Journal is shorter than previous years because we
appeared to be trying for the Guinness Book of Records for the largest Journal
ever. This was becoming a problem for the Printer and the Editor. To obtain the
quality of articles we need is always a problem, as whilst most authors
willingly supply articles when asked I am always aware of not putting extra
stress upon busy people. We also prefer quality to quantity and would prefer
articles from MLSSA members as this is YOUR Journal.
We start with we have another excellent article by the prolific Steve
Reynolds on “Cuttlefish Attacks on Divers”. This well written article is
accompanied by superb pictures from some of the best photographers we use for
our calendars. Steve chose the pictures with care and they show the main
features of the article to great advantage.
Morgan Muirhead, daughter of MLSSA member David, has supplied me with the
article on “Invasive Marine Species”. This subject is explored from a different
point of view. It is very interesting and well researched.
Brian Brock has explored the world of “Early Settlement at Glenelg” as the
first of several short pieces. It is wonderful how a Failsworth Cap can lead a
person on to marine exploration. His articles are all very relevant to the
marine environment and its care.
Steve then adds a
short update to his article published in our 2006 Journal.
DISCLAIMER
The
opinions expressed by authors of material published in this Journal are not
necessarily those of the Society.
EDITING: Philip Hall
PRINTING: Phill McPeake
CONTRIBUTORS: Steve Reynolds
Morgan
Muirhead
Brian
J. Brock
PHOTOGRAPHY etc: As credited in each article
by Steve
Reynolds
I must
say from the outset that I don’t want to put people off of diving with
cuttlefish. It is generally a pleasure to see them during a dive. Since I
always try to encourage people to keep diving despite the perceived threat of
sharks, the last thing that I would want to do is to deter people from diving
with cuttlefish. I do, however, consider that it is important to try to learn
what causes a cuttlefish (or a shark for that matter) to attack a diver, and to
do whatever it takes to prevent such an attack.
ATTACKS
Although I have seen many cuttlefish
during my years of diving (almost 30 years to date), I have only ever been
chased just the one time by a cuttle. That incident occurred some 18 years ago
during an autumn dive at Port Noarlunga South on 7th
April 1989. I can only recall that a largish cuttle caused me to retreat and
try to hide behind rocks a couple of times.
(I had
seen a few cuttles during a short dive at Port Willunga five days earlier
without incident.)
I had
long forgotten about the incident at Port Noarlunga South until 2005 when my
friend Dennis Hutson told me about how he had become the victim of two
cuttlefish attacks. It so happened that the July 2005 issue of Dive Log
featured an article about a cuttlefish attack on a diver. I can still recall
Dennis’s reaction when I showed him the article at the North Haven boat ramp
just prior to a dive on the Norma wreck that same month (17/7/05).
The
article in the July 2005 issue of Dive Log was Sharon Gowen’s
report of how she was attacked by a large cuttlefish during a boat dive at
Split Solitary, near Coffs Harbour, NSW. Although I don’t know the date of
Sharon’s attack, it was on a ‘brisk autumn morning’. (This matches the period
of both Dennis’s attacks and mine.)
Sharon
says that “a huge cuttlefish” which was “about 1m long and nearly half that
wide” attacked her. This cuttle was said to be “quiet” when Sharon first encountered
it at about 14m. It then proceeded, however, to follow Sharon and her two
buddies as they swam away from it. When Sharon looked back at the cuttle, it
was following them at a distance of 10m. It continued slightly higher than the
three divers, “shadowing” them as they went along a ledge. One of Sharon’s
buddies also saw the cuttle following them and thought to herself “it might be
curious”.
All of a
sudden, the cuttle hit Sharon with a “thud” and wrapped its tentacles “around
her head, across her mask and regs”. The tentacles
across her mask blinded her vision. They also pulled her regulator from her
mouth. Her first reaction was to scream. She then grabbed the cuttle’s tentacles and ripped them from her head. She was
able to ‘throw’ the cuttle away from her.
With her
mask full of water, she searched for, and found, her regulator. Trying to
resume breathing on her regulator again, she still could not see clearly. By
this time, one of her two buddies had come to her assistance. The buddy held
Sharon’s hands tightly and accompanied her slowly towards the surface.
Sharon
was able to clear her mask during the ascent. She said afterwards that her
buddy had reassured her and made her feel safe.
But let’s
go back to Dennis’s two attacks. The first attack occurred at Haystack Island,
Yorke Peninsula on 16th April 2005. A smallish cuttle
tried to bite Dennis and he had to fend it off with his cray snare for a
distance of 4 - 5m until it gave up the chase.
Less than one month later, Dennis was
attacked by a larger cuttle at the Norma
wreck off of Semaphore. On 15th
May 2005 a 60cm-long (total length) cuttle had chased him for a distance of 30m
across the wreck. Dennis had to swim hard to keep ahead of the large cuttle,
clubbing it some three times on the head with his dive torch.
When Dennis and I dived at the Norma
on 17th
July that year, we saw several cuttles at the wreck without incident. Dennis
was even able to photograph a large specimen which now features on his website
at
http://home.iprimus.com.au/dghutson/
(under “Photo
Galleries”, “Underwater Realm”, “Octopus and Cuttlefish”). (See page 5)

Cuttlefish at the wreck of the Norma,
Semaphore
(taken by Dennis Hutson)
Dennis’s bad experiences with
cuttlefish were not over yet. He was doing a shallow boat dive at Port Moorowie
on the Yorke Peninsula, SA on 11th March 2006. It was on this autumn
day that Dennis noticed that there was a small female cuttlefish at a range of
about 2m. She approached him and was flashing her colours. Dennis saw this as
an attack pose and he hit her away with a wave of his hand.
This did
not deter her as she came back at Dennis, even angrier, so he backed away from
her. He had moved a good 5m or more from where he had come across her and
thought she might stop. She kept chasing Dennis so he had to hit her on the
head with his cray snare. This only raised the ire within her and she attacked
yet again. Dennis had to hit her extremely hard this time.
She took
off to a nearby ledge and returned with a big male cuttle. This big male was
flashing his colours and heading straight for Dennis, with the smaller female
just behind him. Dennis had to make a ‘beeline’ straight for the boat. As he
swam hard, he checked to see if they were still coming behind him. Fortunately
for him, the male gave up the chase fairly easily.
An
article by Dennis Hutson titled “My Story On The Nasty Cuttlefish Of SA” was
published in our June 2006 MLSSA Newsletter (No.333). The article posed the
question “Has anyone else witnessed this kind of cuttlefish behaviour before,
where one cuttle will seek the assistance of another (bigger) cuttle to attack
a diver?”.
MLSSA
member Dr Brian Brock responded to the article by sending a letter and a copy
of an article to the society. Brian thought that the article “Cuttlefish
Behaviour” by DC Lake in the “South Australian Naturalist”, March 1986, Vol.60,
No.3 answered a few questions.
Brian
suggested that “Colour does not seem to be a factor in attacks” and “The acuity
of vision and memory appear to be the main factors”. He added, “One could not
eliminate sense of “smell” (some chemical factor). Brian suggests that “The
moral seems to be, don’t razz up a cuttlefish”.
Dennis
Hutson has since done further dives at the Norma and the “Barge” during the breeding
season months of March and May. These two dives, however, were without any
further incident. During his dive on the “Barge” on 31st
March 2007, he even managed to feed a dead crab to a medium-sized cuttle of
about 30cm.
During a subsequent dive at the
“Barge” on 23rd
June 2007, a dive buddy demonstrated to Dennis the way a cuttle reacts when a
hand is placed below it. The dive buddy in question was Geoff Prince, who saw humour
in dragging Dennis in close by his tank valve. He demonstrated that you can put
your hand under a male and gently lift it. Dennis then managed to touch the end
of the head of another cuttle. He says that the cuttles are so mesmerised by the mating ritual that you can touch them and
their colours shimmer.
Dennis’s friend John later related
something that he had seen during a TV show. When a (diver’s?) hand was placed
below a cuttle, it would change colouration in such a way that a ‘print’ of the
hand could clearly be seen through the back of the cuttle.

Michael Matthewson placing his hand below a cuttle at Rapid Bay
(taken by Michael Gosling)
NO CUTTLEFISH PATTING
Nicole Strzelecki
from Adelaide Scuba Hove circulated the following email message early in July
2007: -
“I recently received an email from a
concerned club member in regards to patting cuttlefish, particularly now that
they are in breeding season. You may be enticed to stick your hand out and run
your fingers over their soft skin, however, next time consider the
consequences as it can cause the cephalopods to become distressed, as a
result they do not breed and, in some extreme cases, it can result in
death.”
Cuttles are generally thought to die
after mating anyway, but it is best that they have the chance to breed before
they die.
Nicole sent me this cuttle photo taken
by Nathan Menzies (at Whyalla?). (See page 7)

An aggregation of cuttlefish (at Whyalla?) (taken
by Nathan Menzies)
The attacks
on DC (David) Lake had occurred during autumn, on the Anzac Day long-weekend in
1985. They occurred during some BSAC dives off Troubridge Point on the Yorke
Peninsula. They were also reported in the BSAC magazine “Diver” under the title
“Hunted down by a pair of murderous cuttlefish” which was written by my old
friend Bill Mildren.
Bill tells me that, according to his
dive logbook, David Lake and he were diving slightly east of Troubridge Point
on 25th April 1985. They
were down for some 40 minutes and the maximum depth was 16m.
Bill quoted these details from his
logbook: - "Very attractive drop-off and ledges. Clear clean water. Reef. Attacked by two cuttlefish.
Dave was as usual collecting junk and I believe he wanted one of the cuttles
for his pot. He aggravated them."
It seems
that David Lake provoked a cuttlefish by cornering and trying to grab hold of
it. It retaliated by biting him on a finger, right through his glove. Bill says
that he could only watch and laugh. But then another cuttle
bit Bill on the hand too. David says that as they (the divers) swam away
from the scene, the first cuttlefish then proceeded to stalk Bill and himself.
Bill says that they were followed by both cuttles and had to fight them both
off. Both divers received cuts to their hands.
David did subsequent dives with
different buddies over the rest of the holiday long weekend. He suffered from
further approaches by cuttlefish whereas his buddies and other divers did not.
David suggested that the cuttles
“seemed unconfined by territorial boundaries”. They also “demonstrated a memory
capable of retaining information over a period of days with sufficient
discrimination of detail to recognize a particular diver from others wearing
wetsuits, buoyancy vests and masks of the same make and colour”. They also
demonstrated “co-operative behaviour between two apparently unrelated
individuals” and “This co-operation was not the result of a shared threat to
both animals”.
“Learning, memory and “systems of
action wider than dictated by the immediate environment” (Young*) have been
well documented in octopods but little has been
recorded concerning cuttlefish behaviour,” he said.
* “Anatomy of the nervous system of Octopus
vulgaris”
by JZ Young?, 1972, Oxford University Press, NY.
Mike Scotland wrote about cuttlefish
and attacks in his article “Cephalopods – Shimmering, Shredders of the Seas” in
the February 2001 issue of Dive Log. He said “A Giant Sepia
apama* approached (his) dive buddy (Tim)
and postured at close range. It then grabbed his guage
console and tried to take it back to its den. We struggled with the cuttle for
several minutes before it let go of the nice shiny trophy. After the dive, we
noticed that the rubber boot of the console had a 12mm gash in it left by the
powerful beak.” Mike said that he himself tried to bite on the rubber console
as hard as he could but he couldn’t even make a mark.
* (It is
generally assumed throughout this article that the cuttlefish involved are the
Giant Australian Cuttlefish, Sepia apama.)
In the same article, Mike also reports
witnessing a cuttle seizing a small stargazer from the side of a larger
stargazer. He said “a huge cuttle rocketed in, seized the small stargazer with
lightening speed and wrapped it within the extended webbing between its arms”.
It was then seen consuming the little stargazer nearby.
The book “Injuries to Man from Marine
Invertebrates in the Australian Region” by JB Cleland and RV Southcott says
that a small cuttlefish in NSW bit someone in the 1920s. The cuttlefish bite drew
some blood. This attack occurred at Gunnamatta Bay,
Port Hacking, NSW. It was reported in the article
titled “Life of the Tidal Flats” by FA McNeill and T Iredale*
in the Australian Museum Magazine, 2 (8), 1925. The small cuttlefish was said
to be between 12.5 & 17.5cm long.
*
FA McNeill wrote many papers about marine stings. Some of these papers he
co-wrote with Elizabeth Pope. Tom Iredale wrote many
papers about marine molluscs and their stings.
(Strangely
enough, neither “Marine Animal Injuries to Man” by Dr
Carl Edmonds nor the Reader’s Digest “Australia’s Dangerous Creatures” book
seem to discuss cuttlefish at all.)
Tony
Bramley from Whyalla Diving Services says that cuttles will sometimes latch on
to shiny dive gear such as gauge guards, occys*, etc.. outside of the breeding season.
(You can
understand these relatives of octopus grabbing an octopus regulator.)
Two of
the incidents discussed above involved cuttles grabbing either regulators or guage consoles. I, myself, was doing a low-viz dive with
Dennis Hutson at Port Noarlunga reef in the autumn month of May in 2007 when
Dennis witnessed a medium-sized cuttlefish grab at the bunch of car keys
clipped on to my BCD. I was too preoccupied to realize what had happened at the
time. I wondered whether the cuttle believed that my keys were either something
nice to eat or a nice trophy to take back to its den.
Dennis
said that the cuttle was about 30cm total length and
that it retreated to a small den after its unsuccessful attempt to take my car
keys. At least two incidents discussed below involve cuttles grabbing at dive
torches.
It is
perhaps worth mentioning at this point that Alex Gaut said in her article “The
Amazing Giant Cuttle (Sepia
apama)”
(MLSSA Journal, No.11, December 2000) that “Despite their ability to produce an
astonishing range of colour, it is widely believed by scientists that most
cephalopods are colour-blind”.
That same month, I met Ben Gryst (of Bensa Photography &
Imaging) who told me of yet another cuttlefish attack on a diver friend of one
of his own friends. It seems that the diver panicked during the attack and died
as a result of ascending too quickly.

Michael Matthewson offering his regulator to a cuttle at Rapid Bay
(taken by Michael Gosling)
A
cuttlefish at the Rapid Bay jetty attacked Neville Skinner on 21st May 2000. Neville’s dive buddy, Nigel
Muggridge was video filming during the dive when a
mid-sized cuttle started to attack Neville’s wristwatch. The whole incident
lasted at least five minutes and was captured on video. Neville tried
everything to ward off the cuttle, including triggering his camera flash off in
its face.
Here is a still taken from Nigel Muggridge’s video footage: -

Cuttle attacking Neville Skinner at Rapid Bay jetty 21st May 2000
(Taken by Nigel Muggridge)
When
Neville had the opportunity to, he brought out a glove that he carries with him
and put it on his bare right hand to cover his dive watch. After a quick
inspection of Neville’s gloved hand, the annoying cuttle then lost interest.
Neville took up cave diving after this incident.
Neville showed a DVD version of the
video at our July General Meeting. Both a CD and DVD copy of the video are
available for loan from our Society library – mlssa nos. 8028a & 8028b.
Chris Hall recalls two incidents that
have occurred between himself and an aggressive cuttlefish. Although the
precise details are not known, one of them occurred during a dive at the
Dredge, off of Glenelg, about 15 years ago (1992?). Chris was swimming along
the portside keel of the Dredge, close to the bow. A ‘biggish’ cuttlefish
grabbed at Chris’s dive torch. Chris managed to take this photo of the cuttle.

Cuttlefish
at Rapid Bay jetty
(taken by Dennis Hutson)
(Source:
http://home.iprimus.com.au/dghutson/)
The other
incident occurred during a Reef Watch ‘Adopt a reef’ monitoring dive at Hallett
Cove reef on 29th May 2005. In that incident, another
‘biggish’ cuttlefish became aggressive whilst Chris was attempting to take
photographs of something or other else.

The cuttlefish that grabbed Chris’s torch at the Dredge
(taken by Chris Hall)
You may
have noticed an emphasis by me that several (if not all) of the recorded attacks
by cuttlefish occurred during autumn months. I can still recall a dive that I
did at Wool Bay jetty on the Yorke Peninsula in April over 21 years ago. It was
during my dive there on 5th April 1986 that several
largish cuttles behaved aggressively towards me. They would raise two arms
upwards and display a dark-reddish colouring whilst looking directly back at
me, similar to the behaviour shown in the photo below.

Cuttlefish
with two arms raised upwards
(taken at the Barge, Glenelg by Dennis Hutson)
(Source: http://home.iprimus.com.au/dghutson/)
In Gary
Graf’s article “How I learned to get along swimmingly with the Giant Australian
Cuttlefish” in GEO Magazine, March-May 1987, Vol.9, No.1, Gary said “A giant
Australian cuttlefish approaching with two arms raised (is) a type of hostile
behaviour not uncommon during the spring mating season”. A photo of a cuttle displaying this hostility
features in the article. Graf also said that during the “spring* mating season
you may also see a bit of aggression, easily recognisable as the cuttlefish
moves forward with its arms held high as if about to reach out and wrap itself
around you”.
* Certainly, Graf spoke of a “spring
mating season”, and so does Christine Deacon in both “Australia Down Under –
exploring Australia’s underwater world” and “Down Under with the Giant
Australian Cuttlefish”. She mentions a spring mating season, saying that October
was “allegedly the beginning of the mating season for the Australian giant
cuttle”. A caption for one of the article’s photos, however, contradicts this
comment by saying “The giant Australian cuttlefish is seen seasonally in Jervis
Bay, appearing at the beginning of winter. Large numbers of aggressive cuttles
are seen at this time when they are mating”.
Graf says that he usually sees ‘his’
cuttlefish “during the spring and summer months” because “they head for deeper
water during winter”. (Although Graf seems to be from Sydney, Australia, he was
a graduate of Boston University’s School of Communication.) It seems to be
quite the opposite in SA though. Late May is well recognized as being the
beginning of the mating season for the Australian giant cuttle. They seem to
start to come in to shallow waters during autumn, in readiness for the winter
breeding season. Once that the breeding season is over, they
then seem to retreat back to deeper waters. Sharon Gowen’s
attack at Split Solitary, near Coffs Harbour, NSW occurred on a ‘brisk autumn
morning’.
Deacon says that “When (cuttlefish)
raise their tentacles, divers should be careful – as they are large and their
beaks may be dangerous”.
It may well be that autumn is the
danger period when it comes to the possibility of cuttles attacking SA divers,
as indicated by the following table: -
DETAILS OF KNOWN ‘UNPROVOKED’ ATTACKS
ON SA DIVERS
* As discussed earlier, David Lake
provoked a cuttlefish by cornering and trying to grab hold of it. It retaliated
by biting him on a finger, right through his glove. Bill says that he could
only watch and laugh. But then another cuttle bit Bill on the
hand too.
These
same details are grouped in to ‘months’ in the table at the top of page 11: -
As indicated in the two tables, all of
the known ‘attacks’ on SA divers occurred during the three month period of
March to May.
According to the July 2007 issue of
the SODS newsletter (Vol.14, Issue 7,
http://www.sods.com.au/downloads/July07%20magreduced.pdf )
some SODS
members were diving at the Dredge in March 2007 when a new diver was being
“distracted by an amorous Cuttlefish that kept tugging at his torch, attracted
by the light”. This new diver was also struggling at the same time to stop his
mask from fogging up.
Tony Bramley from Whyalla Diving
Services told me that the 2007 cuttlefish-breeding season at False Bay began on
4th May 2007. The taking of all cephalopods, including cuttlefish, from False
Bay, near Whyalla, is not allowed from 1st
March to 30th
September each year.
Many thousands (hundreds of
thousands?) of breeding cuttlefish are expected to gather near Whyalla each
winter. (Some sources say tens of thousands whereas others say hundreds of
thousands.)
Unfortunately, some 450,000 were
harvested there about ten years ago. It was also unfortunate that two fishermen
went and caught 442 of the cuttles at the protected Whyalla breeding grounds in
June 2007. Authorities fortunately caught the two fishermen and confiscated
their catch. The two men then faced serious fishing charges.
I sometimes recorded the odd
cuttlefish sighting in my dive logbook and such recordings were often made
during the autumn months. I have now scanned my logbooks for any references to
cuttlefish sightings. I was surprised to find that I had written a comment
about cuttlefish fighting at Stansbury jetty on 6th
December 1986. Although the Whyalla cuttlefish breeding
season runs from early May until (the start of?) September, I was even
more surprised to find that I had written a comment about cuttlefish mating at
Edithburgh jetty on 29th
September 2001.
SIZE
Sharon Gowens
said that the “huge cuttlefish” which attacked her was “about 1m long and
nearly half that wide”. According to “Australian Marine Life” by Graham Edgar,
the Giant Cuttle, Sepia apama
reaches a length of 800mm. One about that size would appear to be 1m long underwater.
Alex Gaut said in her article “The
Amazing Giant Cuttle (Sepia apama)”
that “Sepia apama
. . ranks as one of the
largest cuttle species in the world”. She goes on to say that they reach a
length of over a metre, saying, “this measurement
includes the arms and tentacles, which can stretch out to almost double the
length of the animal”. She says, however, “Correct scientific measurement uses
the dorsal mantle length i.e. from the front of the mantle between the eyes to
the posterior tip of the mantle”.
She added that “The largest animals
measured in SA had mantle lengths of ~40cm, but in NSW they have been measured
up to ~60cm”. She said that “There is no explanation for this size difference,
it could be different environmental conditions, or possibly different species,
but the research has not yet been done to confirm or deny either of these
possibilities”.
It is generally understood, as
confirmed by Alex, “The largest males are always larger than the largest
females”. In his article “How I learned to get along swimmingly with the Giant
Australian Cuttlefish”, Gary Graf referred to a cuttle that seemed to “stretch
a good 1.5m from tentacle tip to posterior point”.
Tony Bramley from Whyalla Diving
Services says that he himself has seen cuttles to >3kg and almost a metre in
length. He has heard of even bigger ones, which he says would have to be
correct judging by the size of some of the cuttlebones that he has seen.
Two articles by Karina Hall in
“Southern Fisheries” magazine (“Cuttlefish Mysteries” and “The Flamboyant
and Fascinating Lifecycle of the Giant Cuttlefish” both say “The giant
Australian cuttlefish (Sepia apama)
is one of the largest cuttlefish species in the world reaching up to 60cm
mantle length and over 5kg in weight.” “The Flamboyant and Fascinating
Lifecycle of the Giant Cuttlefish” goes on to say “Another point of interest is
that the cuttlefish found in the Black Point area were never as large as the
giants recorded in other regions. The maximum size recorded for a male at Black
Point was 40cm mantle length, as opposed to the 60cm monsters commonly found in
Jervis Bay, NSW”.
It is fair to say then that many large
cuttles will be (or appear to be) a metre or more long.
In the
book “A Guide to Squid, Cuttlefish and Octopuses of Australasia”, by Mark
Norman and Amanda Reid, it says that the Giant Cuttlefish has a mantle length
of “up to half a metre and a total length of one metre”.
Brad
Crouch’s article about the Whyalla cuttlefish breeding season in the “Escape”
section of the 17th June 2007 edition of the
“Sunday Mail” said that “the biggest are about as big as a labrador”
(and just as docile).
QUICK GROWTH
& EARLY DEATH
It has
been suggested that cuttlefish grow extremely quickly and only have a short
lifespan of about 18 months. It has also been suggested that they die at about
this age shortly after mating has occurred. Alex Gaut said in her article “The
Amazing Giant Cuttle (Sepia
apama)”
that “Sick-looking animals have been observed around Black Point (the SA
breeding ground) towards the end of the spawning season. Some have been observed
with the posterior tip of the sepion* protruding
through the mantle, causing the head and arms to droop. Once
in this moribund state, the animals are incapable of withstanding rough weather
and are often found washed up after storms”.
* According to Alex, the sepion is the calcium carbonate cuttlebone, the modified
shell inside cuttles that allows them “to live in the water column like a fish,
able to accurately and quickly control their buoyancy by changing the quantity
of gas in the tiny chambers of the sepion”.
“A Guide to Squid, Cuttlefish and
Octopuses of Australasia” by Mark Norman and Amanda Reid says, “After spawning
most cuttlefish die”. It also says that all cephalopods “Grow very fast . . in a year and are generally
thought to be short-lived, with life spans ranging from a few months to two or
three years”. It then goes on to say, however, that “some large or cold-water
species may live longer” and “Most species of octopus and squid die after
spawning, but other cephalopods may spawn several times”.
Tony
Bramley from Whyalla Diving Services says that cuttles are ‘semelparous’,
(meaning that) they, like salmon, die after spawning. He says that they will
die on the spawning grounds, still trying to mate while they are dying (or
dying whilst they are still trying to mate).
In his
article titled “Cephalopods – Shimmering, Shredders of the Seas” (Dive Log,
February 2001), Mike Scotland said, “Cephalopods have superb growth rates.
Picture the largest Cuttlefish that you have ever seen, well in excess of a
metre in length. It is probably only 6 to 8 months old. The rate of increase in
body size is nothing short of stupendous. Super growth is a relatively common
phenomenon in the marine world”.
AGGRESSION
But let’s
get back to the subject of cuttlefish attacks. Gary Graf said in his article
“How I learned to get along swimmingly with the Giant Australian Cuttlefish”
that although he hadn’t yet heard of any divers being eaten by an aggressive
cuttle, he had “seen a few divers furiously finning the other way, as if such a
fate were a distinct possibility”.
Graf also
described the hunting process of cuttles, saying that “moving quickly forward –
the cuttlefish uses its octet of arms to engulf the victim and shovel it
towards a very sharp, powerful parrot-like (upside down) beak”. He added that,
to his knowledge, “no divers have yet ‘gone’ this way – but I’ve been with a
few who evidently thought such a fate might be on the cards: amazing how fast
one can swim when one really wants to!”
He went
on to say that he had witnessed cuttlefish behaving aggressively towards a
buddy but he was “never sure whether (he) was witnessing a predator bent on
sampling a rubber-skinned alien, or the pass of a would-be lover”.
Could
this last comment be the answer regarding the reason for some cuttlefish
attacks? A successful attacking cuttle has latched on to divers’ facemasks and
regulators as if attempting to lock tentacles, head-to-head, with a mate.
Tony
Bramley from Whyalla Diving Services says that cuttles appear to be very
preoccupied with the mating game during the breeding season and generally
ignore divers completely. They have, however, bitten divers who have grabbed
them (which is fair enough).
One
example of such a provoked attack occurred when Bill Mildren and Dave Shaw were
both diving with a UK BSAC examiner off the Yorke Peninsula (in Aug '94?). Bill
thinks that the examiner interfered with a cuttlefish and it had a go at him.
Quick as a flash, Dave Shaw gave the cuttle a straight left which set it back a
few feet, end of story.
INFORMATION FROM
THE INTERNET
When I
recently did a Google search of the Internet for “cuttlefish attacks”, the response
for "cuttlefish attack"+"diver"was
“Results about 376).”
Video
footage of a cuttlefish attacking a diver at “The Gutter, Bass Point, NSW on 4th April 2004 (04/04/04) can be
seen at
http://www.youtube.com/watchv=Bo53ErZgkRw&mode=related&search
and
http://www.webmunism.com/webmune/Torch
Visit
http://www.youtube.comwatchv=nbOAVlBl6Q4
for some very good video footage of a cuttlefish retaliating
against a nuisance diver. It can also be found at
At some stage of my searching of the
Internet, I found the web page at
http://www.metacafe.com/tags/octopus/
which shows scuba diver Doug Pemberton being attacked on the
head by a large octopus. Octopuses (and squid(s)) are also cephalopods and
therefore related to cuttlefish.
According
to “Observational learning does not explain improvement in predation tactics by
cuttlefish (Mollusca: Cephalopoda)” by Boal J.G., Wittenberg K.M.,
and Hanlon R.T., “Results suggest that odor may serve as a primer for
cuttlefish predatory attack behavior, perhaps by enhancing food arousal and
improving attention”.
for more
details about this paper.
If
you visit
you may be able to see a video of a cuttlefish attacking a
camera.
A web
page titled “Cuttlefish Mystery” by Karina Hall used to be available at
http://www.pir.sa.gov.au/pages/fisheries/rec_fishing/rec90.htm:sectID=299&tempID=10
until PIRSA updated the site. The web page described the aggregation of the
cuttlefish Sepia
apama at
Black Point, tagging studies to determine where they come from and their
complex spawning behaviour. The original source for the web
page was “Cuttlefish Mysteries” by Karina Hall, “Southern Fisheries”
magazine, Winter 2000, Vol.7 No.2, p.10-11.
I visited at the South Australian
Aquatic Sciences Centre Library where the librarian there, Suzanne Bennett,
assisted by providing me with a copy of the original article in “Southern
Fisheries”. She also provided me with a copy of Karen Hall’s earlier article
titled “The Flamboyant and Fascinating Lifecycle of the Giant Cuttlefish”
(“Southern Fisheries” magazine, Summer 1998/99, Vol.6 No.1.).
Suzanne
suggested that “Cephalopod Behaviour” by Hanlon, Roger T. & Messenger, John
B., Cambridge University Press, 1996 (Call no. 594.5H241) might also be of
interest. The book described ‘co-operative hunting’ as “the combined action of
two or more individual predators to secure prey that might otherwise escape”
and went on to say that “there is no unequivocal evidence for such behaviour in
any cephalopod”. The book also says that “Social organisation . . . is weakly
developed or non-existent in cephalopods, except for that related to reproduction.
Cuttlefish (& octopus) are basically solitary animals .
. ”. It also describes reproductive behaviour as “including agonistic behaviour
(i.e. the complex of behaviours that includes fighting, threat, appeasement, . . . (i.e. all behaviour that precedes and
accompanies the sexual act) . . .”.
The
authors of “Cephalopod Behaviour” (Hanlon & Messenger) either haven’t read
David Lake’s comments “They also demonstrated co-operative behaviour between
two apparently unrelated individuals” and “This co-operation was not the result
of a shared threat to both animals”, or they consider either that this
behaviour is “related to reproduction” or that it is “reproductive behaviour”.
Another
paragraph in the book “Cephalopod Behaviour” states that “Neural mechanisms initiating
reproductive behaviour in cephalopods have not been identified but a hormonal
influence on maturation of the gonads has been shown”.
Wells
& Wells performed experiments on octopus in 1959, which showed that the
pathway of physiological control of the reproductive system is:
Light → eye → optic lobe → subpedunculate lobe → optic gland → gonad
“Cephalopod
Behaviour” explains that “The first four links are neural and inhibitory, the
last hormonal and excitory. When the optic gland is
freed from inhibition, it releases a hormone . . . that causes the gonad to
grow. In Sepia it seems that there may be more than
one hormone involved”.
When I left the South Australian
Aquatic Sciences Centre Library, I was able to have a quick word with Scoresby
Shepherd, our Patron, about my research on cuttlefish attacks. Scoresby was
surprised to hear, despite his many years of diving experience, that anyone
would be fearful of being attacked by a cuttlefish. He thought that there seems
to be very little on record regarding any actual injuries being caused by a
cuttlefish bite, and I agreed. We also agreed that any retreating diver would
probably be mostly fearful of the unknown when it came to the threat of being
bitten by a cuttlefish. But what about some of the incidents reported above?
David Lake and Bill Mildren, for example, were both bitten on their hands.
A few ‘provoked’ attacks have been
described above, but consider this table summarising
the ‘unprovoked’ attacks described above: -
I can’t
say for certain that the ‘attack’ in the 1920s was ‘unprovoked’ because I don’t
have any idea what may have happened. There was only one injury from all of the
other incidents. Sharon Gowens could easily have
suffered more were it not for the assistance of her dive buddy. The friend of
Ben Gryst’s friend apparently suffered the ultimate
fate when he panicked and surfaced too quickly, although this hasn’t been
confirmed.
Some
vital information about cuttlefish attacks came through just after the
completion of this article. It came through in the form of an email message
from Antony King who described some three separate attacks on two different
divers during the one dive in the month of May: -
“Hi
Steve, I have been chased by Cuttlefish... I think it was 12th May 2007 i.e. the start of
breeding season (I think) at Seacliff Reef, entering the water at 11.30.
They're usually quite placid, but this time (on the same 100-minute long dive),
one had a go at my camera, and another at my torch. I think the one that had a
go at my camera was attracted to the red lens surround. It didn't chase me, but
I have a picture of lots of tentacles! The other one actually followed me for
some way. I noticed it when I felt it tugging the head of my canister torch,
which was hanging below me (I had neglected to clip it off). I think it was
attracted to the bright blue surround of the torch. It kept following me for a
while, even as I had ascended around 3-4 metres. I came to the conclusion that
it was breeding season, and (that) the colours (of the torch) attracted (it).
On the same dive, someone with brightly coloured fins found a cuttlefish
attached (to their fin). At Seacliff Reef, I think there are less potential
'partners' than at Point Lowly, etc., so the Cuttlefish will try to mate with
pretty much anything. Whilst it could be a bit frightening for some divers
(esp. new ones), I think the easiest way to make the cuttlefish lose interest
is to give it a gentle nudge with one's fins. It also means that the diver’s
face, etc.. is the furthest
point from the cuttlefish.
Cheers,
Antony.” Many thanks go to Antony for this information. (See picture below.)

Cuttle trying to latch onto Antony King’s camera
(taken by Antony King)
POISONOUS?
“A Guide to
Squid, Cuttlefish and Octopuses of Australasia” by Mark Norman and Amanda Reid
says “Cephalopods’ mouths have a hard beak, resembling a parrot’s beak, which
they use to kill or paralyse prey by injecting poisonous saliva”. (It also says
that octopus “inject
poisonous saliva”.) Again, it says that some cephalopods use poisons as a means
of defence to protect themselves.

Aggressive cuttlefish on Seacliff Reef
(Photo by Antony King)
MORE ABOUT THEIR
BEAKS
The beak of any cephalopod is part of
what is called the ‘buccal mass’. The ‘buccal mass’
includes the beak, radula and the salivary system. According to the book
“Cephalopod Behaviour”, “The cephalopod ‘buccal mass’ is a large and complex
structure, comprising the beak with its associated muscles, the radula, the
salivary papilla, the salivary glands with their ducts and the sub-mandibular gland. It lies in front of the brain, in the
centre of the arms. The beak is chitinous and its
musculature generally massive (Kear, 1994) so that it
can be a formidable weapon, as anyone who has been bitten by a cephalopod will
know”.
Further, “The radula is a toothed
ribbon that moves back and forth like a rasp . . . It carries food towards the
oesophagus, and in some octopods it is known to be
involved in the initial stages of drilling holes in the shells of molluscs and
the exoskeleton of crustaceans.
It seems, however, that the poisonous
saliva mentioned above stems from the salivary papilla, which carries the duct
from the posterior gland. According to “Cephalopod Behaviour”, “The essential
organ for drilling . . is the
salivary papilla which carries the duct from the posterior salivary gland
(PSG). The papilla and the eversible tip of the duct bear small teeth and the
secretions of the PSG are delivered precisely to the drilling site”.
It goes on to say that “In Sepia
officinalis . . this PSG secretion contains a
‘cocktail’ of other substances, including dopamine and serotonin, toxins, proteolytic enzymes and chitinases.
The principal toxin is cephalotoxin”.
According to Karen Hall’s article “The
Flamboyant and Fascinating Lifecycle of the Giant Cuttlefish”, cuttlefish “have
extremely strong beaks, similar to a parrot, which they can use to crack open
hard-shelled animals like crabs, and a tooth lined tongue for rasping away at
food held in the arms”.
Dennis Hutson says that the attack
pose is when the cuttles point straight at you and close up their tentacles.
When they are only 30cm from your face, they lunge at you, wrap their tentacles
around your head and bite you with their very sharp and very hard beak. A few
divers have had their equipment bitten.
The view
shown in the next photo is the last thing a victim sees before being bitten by a
cuttle.

The last thing a victim sees before being bitten by a cuttle
(taken by Michael Matthewson at Seacliff)
One
cuttlefish 'attack' that resulted in a diver being bitten occurred at Rapid Bay
jetty around 2003-4. Hank van der Wijngaart was diving from his boat anchored
about 50m north of the eastern end of the ‘T’ end of the jetty. Around the
centre of the ‘T’, he found a cuttle tangled in fishing line and proceeded to
free it. He didn't really expect a wild animal to realize that he was helping
it. As he tried to free it, it gave him a bite. The bite wasn’t severe but it
was a bit of a shock or a surprise to Hank more than anything. He did, however,
manage to free the cuttle which then swam away. Hank says that his injury from
the cuttle bite was not serious and that he had forgotten about it by the time
he had surfaced.
I don’t know what time of the year Hank’s attack occurred and it’s not
really relevant since the cuttle was only protecting itself from a large
diver creature.
MORE ABOUT THEIR ARMS
According to “The Flamboyant and
Fascinating Lifecycle of the Giant Cuttlefish” by Karen Hall, “Cuttlefish have
eight sucker lined arms growing from the front of their head, similar to squid.
They also have two longer tentacles, which are kept tucked away in pouches
underneath their eyes. They shoot these tentacles out in a rapid whip-like
motion to seize their prey within their arms while they consume it”.
MORE ABOUT THEIR
DENS & TERRITORIALITY
Also according to “The Flamboyant and
Fascinating Lifecycle of the Giant Cuttlefish”, “Sepia
apama have been described by divers as
being solitary animals, inhabiting caves and overhangs. The males are very
territorial, and fiercely protect choice dens in order to attract females
looking for a place to lay their eggs. Even in other regions of South Australia
such as near Edithburgh in Gulf St Vincent, male cuttlefish have also been
reported to occupy and guard dens”.
This may sound strange when
considering the mating behaviour that occurs in the waters of upper Spencer
Gulf each winter, but that sort of behaviour (large spawning aggregation)
occurs in that area only and nowhere else. As Karina Hall says in her article
titled “Cuttlefish Mysteries”, “Nowhere else does such a large spawning
aggregation of cuttlefish occur. Generally, the
individuals of most cuttlefish species are solitary animals occurring in low
densities over very large areas. Numbers of the giant Australian cuttlefish in
other coastal areas do increase during the spawning season. However, they do
not reach the extremely high densities commonly observed in the waters adjacent
to Black Point (near Whyalla, upper Spencer Gulf)”.
As already mentioned above, “The
Flamboyant and Fascinating Lifecycle of the Giant Cuttlefish” says that
“(Elsewhere across their distribution,) Sepia apama
have been described by divers as being solitary animals, inhabiting caves and
overhangs. The males are very territorial, and fiercely protect choice dens in
order to attract females looking for a place to lay their eggs”. It goes on to say
“The cuttlefish of the Black Point area, however, behaved in a very different manner.
Instead of males guarding a den or territory, they fiercely guarded their
chosen female”.
PROTECTION
FROM CUTTLES?
If
cuttlefish are more of a threat than we realize, we may soon have to start
wearing a shark shield kind of device (a Sepia Shield?) to protect ourselves
from them. With a Shark Shield attached to one leg and a Sepia Shield attached
to the other leg, we will start to look like an octopus, or similar creature,
ourselves. But seriously, it should never come to that.
The
August 2007 issue of the SODS newsletter featured a photograph of Michael
Matthewson with his face right up next to a largish cuttle. It seems that the
cuttle didn’t mind the close attention by Michael at all. (See picture on page
18.)
Chris
Deacon’s two articles said, “The colour patterns on the giant
Australian cuttlefish suggests it is curious as it approaches a diver
and lets her stroke its tentacles”. We need to show cuttlefish more respect,
however, by leaving them alone and not harassing them in any way though, and we
probably shouldn’t be attributing human emotions such as ‘curiosity’ and
‘friendliness’ to wild creatures such as them.

Michael Matthewson with his face right up next to a largish cuttlefish at
Rapid Bay
(taken by Michael Gosling)
WHYALLA CUTTLEFISH SEASON
The
annual cuttlefish-spawning season at the top of SA’s Spencer Gulf runs from May
until September each year. Phone any of: - 8645 7900, 1800 088 589, or 8682 4688,
or visit either of
http://www.whyallacuttlefish.com/cuttlefish/index.htm,
http://www.whyallacuttlefish.com/cuttlefish/Cuttlefish%20A4%20brochure.pdf,
www.tep.com.au
or
to find out more about the cuttlefish. Phone Whyalla Diving
Services on either 8645 8050 or 8644 1141, or contact them at
to
arrange for a dive with the cuttlefish.
Whyalla
Diving Adventures
(www.whyalladivingadventures.com)
can be contacted on 0418804421.
You can
view some cuttlefish information on the PIRSA website at
http://www.pir.sa.gov.au/fisheries/recreational_fishing/target_species/cuttlefish
including the following topics: - What Are They, Habitat, Movement, Feeding,
Predators, Reproduction, Catch History, Closures, Catch
Limits & Legal Lengths.
You
can also view the PIRSA pamphlet “Cuttlefish Closure – Spencer Gulf” (October
2006) at
http://www.pir.sa.gov.au/__data/assets/pdf_file/0019/13096/cuttlefish1.pdf
.

Cuttlefish on Seacliff Reef
(Photo by Antony King)
ACKNOWLEDGEMENTS:
My thanks
to everyone who assisted me with this article, especially Dennis Hutson, Brian
Brock, Tony Bramley, Chris Hall, Neville Skinner, Scoresby Shepherd, Bill Mildren,
Hank van der Wijngaart, Ben Gryst, Michael
Matthewson, Kevin Smith and Suzanne Bennett (the librarian at the South
Australian Aquatic Sciences Centre Library). Thanks
again to Dennis, Chris, and Michael M, along with Michael Gosling, Nathan Menzies and Nigel Muggridge for
allowing me to use their cuttlefish photos.
REFERENCES:
“My Story On The Nasty Cuttlefish Of SA” by Dennis Hutson, MLSSA
Newsletter, June 2006, No.333.
“Attack of the Killer Cuttlefish” by Sharon Gowens,
Dive Log, July 2005, No. 204.
“Cuttlefish Behaviour” by DC Lake,
South Australian Naturalist, March 1986, Vol.60, No.3.
“Cephalopods – Shimmering,
Shredders of the Seas” by Mike Scotland, Dive Log, February 2001.
“The Amazing
Giant Cuttle (Sepia apama)”
by Alex Gaut, MLSSA Journal, No.11, December 2000.
“Australia Down Under – exploring Australia’s underwater world” by
Christine Deacon, 1986, Doubleday Australia P/L, ISBN 0 86824 241 1.
“How I learned to
get along swimmingly with the Giant Australian Cuttlefish” by Gary Graf, GEO
(Australasia’s Geographical Magazine) March-May 1987, Vol.9, No.1.
“Down Under with
the Giant Australian Cuttlefish” (an excerpt from “Australia Down Under –
exploring Australia’s underwater world” by Christine Deacon), GEO Magazine,
March-May 1987, Vol.9, No.1, (along with Gary Graf’s article “How I learned to
get along swimmingly with the Giant Australian Cuttlefish”).
“A Guide to Squid, Cuttlefish and
Octopuses of Australasia” by Mark Norman and Amanda Reid, Gould League of
Australia/CSIRO Publishing, 2000, ISBN 0 643 06577 6.
“Injuries to Man from Marine Invertebrates in the Australian Region” by JB
Cleland and RV Southcott, Commonwealth of Australia, 1965.
“Life of the
Tidal Flats” by FA McNeill* and T Iredale in the Australian
Museum Magazine, 2 (8), 1925. (This article is listed in the
Australian Venom Compendium website at
http://barney.asap.unimelb.edu.au/avru/compendium/scripts/avru-bib.php3?pubid=PR0001505.)
The July 2007
issue of the SODS newsletter, Vol.14, Issue 7,
(http://www.sods.com.au/downloads/July07%20magreduced.pdf
)
http://www.metacafe.com/tags/octopus/
- the
web page which shows scuba diver Doug Pemberton
being attacked on the head by a large octopus.
http://barney.asap.unimelb.edu.au/avru/compendium/
- the website for the
Australian Venom Compendium.
“Cephalopod Behaviour” by Hanlon, Roger T. & Messenger, John B.,
Cambridge University Press, 1996.
Suzanne Bennett, the librarian at
the South Australian Aquatic Sciences Centre Library also suggested that the
following entries from the library’s catalogue might be of
interest: -
“Cuttlefish
(Sepia apama): Fishery Assessment report to PIRSA for the Marine Scalefish
Fishery Management Committee. 2002” (South Australian Fisheries Assessment
Series 01/09 SARDI Internal Report No.139) by Hall, Karina C. (South Australian
Research and Development Institute) - Call no. 639.2(06)S8.
“The Life history and fishery of a spawning aggregation of the giant Australian
cuttlefish Sepia apama” by Hall, Karina C. (University of Adelaide) - Thesis
submitted to the University of Adelaide for the degree of Doctor of Philosophy,
2002. Call
no.639.27H177.
“Estimated abundance and biomass of the unique spawning aggregation
of the Giant Australian Cuttlefish
(Sepia apama) in northern Spencer Gulf, South
Australia (SARDI Aquatic Sciences Publication No.
RD05/0012-1 SARDI Research Report Series Number 97 - Report to Coastal
Protection Branch, Department for Environment
and Heritage, South Australia) 2005, by Steer,
M.A. Hall, K.C. (South Australian Research and Development
Institute (Aquatic Sciences) ). Call no. 639.2(06)S8.
“Fisheries biology of the cuttlefish,
Sepia apama Gray, in South Australian waters” (FRDC
Final report) by Hall, K. C. Fowler, A.J. (South Australian Research and
Development Institute Fisheries Research & Development Corporation FRDC
Project 98/151), 2003.
Call no.639.2(06)S723.
“Dynamics of
the mating system of the giant Australian cuttlefish, Sepia apama Gray” by
Hall, K. C. & Hanlon, R.T. (Bulletin of
Marine Science Vol.71(2) 2002; p.1125). BRIEFS Hall2
(The South
Australian Aquatic Sciences Centre Library (reference only) is located at 2
Hamra Ave, West Beach S.A. 5024. The hours
of opening for the library are: -
Tues. 1 - 4pm,
Wed. 9am –12noon + 1 - 4pm, Thurs. 9am –12noon. Contact details are: -Phone:
82075423, Fax: 82075422, Email: bennett.suzanne@saugov.sa.gov.au
.
Visit
for more details.)
by Morgan Muirhead
Introduction
Australian inshore marine waters are among the world's most biologically diverse. That marine biodiversity
has become a prominent topic for debate in the international legal regime over
the past few decades perhaps should not surprise us given that “of the
thirty-five phyla found in nature, all but one is found in the marine
environment.”1
Habitat destruction and invasive species are recognised
as the two greatest causes of biodiversity reduction.
While there are many ways in which invasive marine
species can be introduced to Australian waters, the large majority are
established through the discharge of ships’ ballast water. Ballast water is the
sea water that ships take on at port either by gravity or through pumping, in
order to maintain stability and enhance voyage safety. At any given moment,
approximately 3,000 different marine species are being transported in the
ballast water tanks of ships around the world.2
As travel times between ports reduce and the range of
overseas ports trading directly with Australia increases, unprecedented levels
of invasive marine species are brought to our shores.
International legislation designed to prevent and control
the spread of invasive marine species exists but is fraught with gaps, overlaps
and inconsistencies ranging from problems with terminology, scope and taxonomic
coverage to weaknesses in the provisions regarding eradication and control. On
this latter point, while if effective this emphasis would be logical and cost
efficient, the available evidence suggests that prevention is not always
achievable and thus will never obviate the need for fully funded aggressive,
timely and expert eradication protocols.
As vessels are the primary vector for the introduction of
invasive marine species, a particular focus of this essay is the International
Convention for the Control and Management of Ships’ Ballast Water and Sediments
2004 (BWC).3
Furthermore, a consideration of the relationship between invasive species
legislation and the multilateral trading system is crucial to understanding the
limitations currently facing regulation of invasive marine species.
Invasive alien species (IAS) are those species,
sub-species, or lower taxon introduced outside their normal past or present
distribution whose “establishment and spread threaten ecosystems, habitats or
species with economic or environmental harm.”4
IAS are introduced through trade intentionally (imported products such as fish
for aquaculture) or unintentionally (by-products, parasites of traded products,
hitchhikers and stowaways in vessels).
Between 150 and 200 million tonnes of ballast water from
overseas locations are discharged inside Australia’s territorial seas every
year, resulting in more than 250 introduced marine species now being
established in Australian waters.5
Devastatingly, the ecological consequences of such
exotic species are largely irreversible. The discovery in March 2002 of the
invasive seaweed Caulerpa taxifolia
in West Lakes and the upper Port River is a South Australian example of an
invasive marine pest which has begun to threaten local biodiversity through the
exclusion of native species.
International Legislative Regime
At least forty-two treaties dealing with environmental
issues, the marine environment and international quarantine refer to the
regulation of IAS.6
Given the recent increases in the dimension of the IAS
problem, it is widely recognised that isolated action by States is not enough
to adequately manage and prevent their introduction. Two key provisions dealing
with invasive marine species are contained in the 1992 Convention on Biological
Diversity (CBD) and the United Nations Convention on the Law of the Sea 1982
(UNCLOS). Article 8(h) of the CBD states that each contracting party “shall, as
far as possible and as appropriate, prevent the introduction of, control or
eradicate those alien species which threaten ecosystems, habitats or species.”7
Whereas Article 196 of UNCLOS requires parties to take all measures necessary
to prevent, reduce or control pollution of the marine environment resulting
from the intentional or accidental introduction of alien or new species to a
particular part of the marine environment, which may cause significant and
harmful changes thereto.8
Both the CBD and UNCLOS fail to provide any guidance on how either of these
objectives should be achieved. Further, the broad and ambitious style of the
provisions hinders States from clearly understanding the nature of their
international obligations.
Problems with the International Regime
IAS terminology presents particular challenges for
scientists, policy makers and lawyers. Definitions are used in legal
instruments to provide an agreed meaning for a particular term, clarify scope
and provide legal certainty and consistency.9
However, legal instruments such as the CBD and UNCLOS use variable terminology,
sometimes inconsistently and without adequate definitions. The term
‘accidental’ is widely used as a synonym for unintentional. However, many
consider this terminology to be inappropriate in the context of pathways where
risks associated with the introduction of species are well known and possible
damage is not unforeseeable.10
Sanitary and Phytosanitary instruments use ‘pest’ and
‘weed’ terminology, preferring to avoid terms like ‘alien’ and ‘invasive’ as
they are considered too emotive.11
Perhaps one of the greatest drawbacks of the international legal regime is the
lack of agreement on a precise definition of exactly what constitutes an IAS.12
Further complicating the legal
instruments is that they are consistently qualified by phrases such as
‘significant harm’, ‘as far as possible’, ‘to the extent possible’ that give no
direction on how significant the harm needs to be to amount to an environmental
threat or how the appropriateness of measures is to be determined.13
Terms such as these give rise to inconsistency in implementation.
Taxonomic scope presents another area of discrepancy.
Biological invasions can be generated by all taxonomic groups and at all
taxonomic levels. Only the CBD adequately covers all aspects of IAS as they
relate to all levels of the biodiversity hierarchy. While the CBD addresses
alien and new species separately, UNCLOS groups alien and new species together.
‘New’ is a reference to living modified organisms, which arguably do require
different legal treatment to alien species.
Further, there is virtually no legislation relating to
marine introductions generated by land-based activities, including through
sewage and runoffs, other than those that come within the scope of UNCLOS (Art.
194). Again, no substantive guidance has been developed to facilitate
implementation.14
The CBD obligation has three components: prevention,
eradication and control. In practice, these components should be part of a
broader framework that starts even before prevention and continues beyond
control and restoration of an ecosystem.15
Elements of this broader practical framework should ideally include
establishment and maintenance of a knowledge base on alien species, continuous
monitoring, feedback and policy review. Currently there are no binding
instruments that explicitly link IAS with the rehabilitation of degraded
ecosystems. While the Ballast Water Convention recommends that port States
undertake biological monitoring in their ports and implement early warning and
information dissemination systems, other multilateral environment agreements do
not usually distinguish ‘eradication’ from ‘control’ or provide guidance on
implementation. The Secretariat of the CBD has highlighted that this gap
between ‘eradication’ and ‘control’ is most marked for the marine environment.16
An exception to this is The Bern Convention, which is unique for its suite of
recommendations on eradication and control of named alien species, such as Caulerpa
Taxifolia.
The pathways by which marine species are introduced may
be intercontinental, intracontinental, transboundary or domestic. Invasion processes ignore
political boundaries between countries and their states. Consistent with the
ecosystem approach developed under the CBD (which is a strategy for maintaining
an ecosystem as a functional, dynamic unit), legal frameworks need to support
coordinated approaches and consultation between different jurisdictions on
prevention, early warning and mitigation. The CBD does address this issue in
relation to marine and coastal biodiversity but not with enough specificity. It
urges “particular attention to transboundary effects”
of alien species and genotypes that threaten marine ecosystems, habitats and
species.17
Existing international environmental law is generally
under-developed and lacks rules for the possible liability of States for damage
related to invasive marine species. The lack of clear rules is a serious flaw
because it means that biodiversity-related prevention and control obligations
are not underpinned by a deterrent element.18
Further, none of the existing legal instruments
provide any remedies for victims of environmental damage. Article 14(2) of the
CBD requires parties to examine issues of liability and redress but does not
provide any further details.19
Important issues such as these ultimately require a
much greater degree of precision in order to be effective.
In common with most other areas of international law,
there is an inconsistent level of commitment and obligation. Many treaty
obligations have not been uniformly adopted by all interested or affected
parties, for example, the USA is not a party to the CBD.20
Conventions such as the CBD and UNCLOS merely indicate preferred outcomes and
do little in the way of providing guidance on how to accomplish their
objectives, and as a result many of the international provisions relating to
IAS remain largely ineffective.
On a more positive note, institutional linkages between
relevant marine organisations have expanded over the past decade, promoting
coordination of legal drafting processes to ensure greater consistency and
efficiency. The International Maritime Organisation (IMO) has developed
institutional links with the World Maritime University, the UN Train-Sea-Coast
programme, the global shipping and port industries and international
environmental non-governmental organisations in order to coordinate activities
on a global scale.21 At
a regional level, Australia and New Zealand show increasing cooperation between
regulatory agencies on the development and review of legal instruments and
quarantine health standards. Australia’s ballast water requirements were
developed with cross-industry group support.
Since 2001, under the Australian Ballast Water Management
Requirements (ABWMR)22,
all ballast water from outside Australia’s territorial sea (generally 12
nautical mile limit) must be managed to make it low-risk to the marine
environment before being discharged. Ballast water categorised
as presenting a high-risk of introducing exotic marine species is all salt
water from ports or coastal waters outside Australia’s territorial sea. The
ABWMR, like most other national ballast water requirements, implement the
guidelines of the IMO which emphasise ballast water exchange outside of coastal
waters. Compliance by vessels arriving to Australia is better than ninety-nine
percent for the roughly 12,500 annual voyages.23
International Convention for the Control and Management
of Ships’ Ballast Water and Sediments 2004 (The Ballast Water Convention – BWC)
On 13 February 2004, the IMO adopted the BWC, which is
the first international legal instrument to attempt to address the risks posed
by ballast water. Australia has been prominent in providing drafting and
support for the BWC, and was the first country to notify its intention to sign
the Convention, which aims to encourage the development of standards to prevent
ballast water from transporting aquatic IAS around the globe.
Obligations are imposed on both flag states and port
states. The BWC establishes default concentration-based discharge standards,
which a ship flying the flag of a State Party must meet by a certain date, and
requires State Parties to apply the BWC to nonparties as a port entry
condition, ensuring they have no more favourable
treatment than State parties. Ships must develop ballast water management
plans, maintain a ballast water record book, undertake certain ballast water
management measures, and eventually comply with the concentration-based
discharge limits.24
The significance of the BWC is that it takes the
international community beyond outdated focus on protection of the marine
environment and moves towards biodiversity protection, essentially by
addressing the power imbalance between flag states and port states. However,
until the convention comes into force (when thirty states covering at least
thirty-five percent of the world’s gross tonnage have unreservedly become
parties to the Convention25)
only basic interim precautions, together with a complex surveying and
certification system, will be imposed, and furthermore, these restrictions will
only be imposed if they do not cause delay or deviation for ships.26
Much more legislative and regulatory work needs to be done on the BWC framework
before it becomes binding. For now, it is important in its own right because it
heralds broader institutional change at the IMO, which is increasingly being
forced to balance its traditional emphasis on ship and crew safety with
biodiversity concerns.27
There are several ways in which the IMO has shifted away
from its traditional approach to pollution prevention. Importantly, the
preamble of the BWC acknowledges the threat that ballast water poses to the
conservation and sustainable use of biological diversity.28
A central provision of the convention is that it explicitly regulates the
discharge of those organisms and pathogens that “may… impair biological
diversity”, suggesting an expanded role for the IMO as the regulatory authority
of the Convention.29
Definitions include the use of the arguably more restrictive term ‘harmful
aquatic organisms and pathogens’ as opposed to IAS, and these are made with
greater reference to biodiversity protection than pollution prevention.30
But some vague qualitative phrases as witnessed in the UNCLOS and CBD are used,
for example Article 4.2 requires each party, “with due regard to its particular
conditions and capabilities”, to develop national ballast water management
policies and promote attainment of the Convention objectives.
Inspection, enforcement and sanction provisions have been
incorporated into the BWC to ensure there is some ability to deter ships’
violation of Convention standards. Article 9 provides that port states have the
right to inspect flag states’ ships, in order to verify that the ship has a
valid International Ballast Water Management Certificate, inspect the Ballast
Water record book, and sample the ballast water in accordance with IMO
guidelines. This authorisation of compliance sampling rather than merely a
paper examination is a crucial step that should enforce compliance with the
Convention. Flag, coastal and port states are required to establish sanctions
for violations. Article 8 requires that where there has been a violation,
parties adopt sanctions that “shall be adequate in severity to discourage
violations of this Convention wherever they occur”. The legislative drafters
have offered no more specificity on the type of sanction to be imposed. Port
and coastal states have authority not only to deliver information regarding a
violation to the flag state, but also to institute enforcement proceedings.31
Ultimately the BWC envisages an expansion of port state control. Flag state
control is increasingly outdated and must be complemented by, and in some
situations give way to, coastal and port state control.
Unfortunately the BWC, although not yet in force, appears
for the most part to be crippled by the same gaps and imprecision of the CBD
and UNCLOS. However, producing a more aggressive legal instrument, with more
immediate impact, may alienate the shipping industry and decrease the
likelihood of ratification, hence rendering the whole process static.32
However, the alternative is that the environmental benefits of the BWC will
remain limited and the major risks presented by the introduction of marine
species will persist. The only way to properly address ballast water issues in
the long term is to hold the shipping industry accountable. Any heavy solid or
liquid can serve as ballasting material, the only reason ships exclusively
employ sea water is for operational and economic convenience. Hence ship
construction laws should arguably be amended so that all new ships built cannot
physically take on any water from the marine environment. As it stands,
assuming timely entry into force of the BWC, the concentration-based ballast
water performance standards will come into effect between 2009 and 2016,
depending on vessel class, size and construction date. By 2016 all ships should
have the required technology to treat ballast water.33
The dilemmas posed by the shipping industry are
complicated by the fact that invasive marine species are introduced through
multiple vectors – not only through ballast water and sediments but also
through hull fouling and anchor chains. Organisms attach themselves onto the
hull and anchor chains of the ship at one location and detach at another.34
Unfortunately the BWC excludes other ship-associated
vectors in order to concentrate specifically on marine pest introduction by
ballast water and sediments.
Biodiversity versus Free Trade
Recent increases in global trade, spurred in part by free
trade agreements, have driven the problems associated with invasive alien
species to devastating levels. Because alien species move through international
transport and trade pathways, national measures to prevent or minimize risk of
unwanted introductions have implications for the multilateral trading system.35
The World Trade Organisation (WTO), primarily through the Agreement on the
Application of Sanitary and Phytosanitary Measures
1995 (the SPS Agreement), sets out binding principles and standards to be
followed in national measures. These principles are focused on animal, plant
and human life and health/food safety, and include no specific provisions
addressing ecosystem function. Where no international standard exists, the
State concerned must justify a national measure as it affects international
trade through scientifically-based risk assessment, essentially forcing the
State to protect the right to trade before protecting their biodiversity.
The SPS agreement thus hinders the development of
environmental legislation based on the precautionary
principle, which dictates that
cost-effective preventive measures should not be held back because of
scientific uncertainty where there are threats of irreversible or serious
damage.36
Our limited understanding of invasive marine species is a problem compounded by
the potentially long lag period between introduction and invasion.37
Even so, there is little doubt that ballast water has already contributed to
irreversible alteration of many ecosystems worldwide. The CBD endorses the use
of the precautionary principle
as an appropriate standard in the administration of IAS, and indeed reference
to the principle is contained in the Convention’s preamble, yet the stance
taken by the WTO makes application of the principle virtually impossible.38
In order to address this issue, environment and trade policies need to be
mutually supportive with a view to achieving sustainable development. Some sort
of enlargement of existing sanitary and phytosanitary
instruments should take place in order to build a closer interface with
biodiversity parameters.39
Conclusion
The great uncertainty regarding the impacts of existing
marine IAS makes the already considerable challenge of drafting domestic or
international laws that are capable of adequately addressing the introduction
of IAS even more difficult. Efforts undertaken by the CBD, UNCLOS and BWC in
formulating principles provide a strong basis for incorporating core concepts,
such as the precautionary principle, into the IAS regulatory framework. That
said, a key element of strategic planning must be the
review of existing legal instruments and practices to resolve issues with
regulatory gaps and loopholes. Essentially the international legal regime for
the regulation of IAS remains ineffective due to the ambiguous nature of the
environmental treaties and the lack of specificity in relation to exactly how
States should implement measures to achieve treaty objectives. Inconsistencies
in terminology, scope, political boundaries, weakness in eradication and control
provisions, and lack of liability and redress provisions further complicate the
problem. The BWC highlights a fundamental change in the role of the IMO towards
biodiversity protection. While the BWC is a significant step forward in
representing the increasing concerns among the global community about the
environmental impacts of ballast water, it is crucial that this Convention
comes into timely force. A major barrier to implementation of the precautionary
principle is presented by Australia’s free trade obligations under the SPS
Agreement. This conflict needs to be overcome by amending the SPS Agreement so
that it accommodates greater biodiversity protection. We cannot afford to compromise
the biodiverse flora and fauna that inhabit our
oceans, if not for our own continued existence, then purely for their intrinsic
value.
Bibliography
Articles/books/reports
Bates, Gerry, Environmental
Law in Australia (5th
ed, 2002).
Beeton RJS, Buckley Kristal I, Jones
Gary J, Morgan Denise, Reichelt Russell E, Trewin Dennis, ‘Independent Report to the
Australian Government Minister for the Environment and Heritage – State of the
Environment Report’ (2006) Australian State of the Environment
Comittee.
Firestone, Jeremy and Corbett, James J, ‘Coastal
and Port Environments: International Legal and Policy Responses to Reduce
Ballast Water Introductions of Potentially Invasive Species’ (2005) 36 Ocean
Development and International Law
291-316.
Firestone, Jeremy,
‘Dilemmas and Dimensions of Non-Indigenous Organisms and Pathogens in the
Marine Environment: A Sea Change’ (2006) 9 Journal of
International Wildlife Law and Policy 123-132.
Leverenz, Renae, ‘Legislative Control
of Invasive Species in Australia’ (2002) 13(1) Polemic
28-32.
Riley, Sophie, ‘Invasive
Alien Species and the Protection of Biodiversity: the Role of Quarantine Laws
in Resolving Inadequacies in the International Legal Regime’ (2005) 17(3) Journal
of Environmental Law 323-359.
Secretariat of the
Convention on Biological Diversity, ‘Review of the Efficiency and Efficacy of
Existing Legal Instruments Applicable to Invasive Alien Species’ (2001) CBD
Technical Series no.2.
Tsimplis, Michael, ‘Alien Species Stay Home: The International
Convention for the Control and Management of Ships’ Ballast Water and Sediments
2004’ (2005) 19(4) International Journal of Marine
and Coastal Law 411-445.
Treaties
Convention on Biological Diversity, opened for signature 5 June 1992, ILM (entered into force 29 December
1993).
International Convention for the Control and Management of Ships' Ballast
Water and Sediments, opened for signature 13 February 2004, ILM (not yet in force).
United Nations Convention on the Law of the Sea, opened for signature 10 December 1982, 1833 UNTS 3 (entered into force 16
November 1994).
Other Sources
Australian Quarantine and Inspection Service, ‘Australian Ballast Water
Management Requirements’ (2001)
<www.affa.gov.au/corporate_docs/publications/html/
quarantine/ballast_water/Australian_BW_Requirements.pdf>
at 5 March 2007.
REFERENCES
1 Jeremy Firestone,
‘Dilemmas and Dimensions of Non-Indigenous Organisms and Pathogens in the Marine
Environment: A Sea Change’ (2006) 9 Journal of
International Wildlife Law and Policy 123, 125.
2 Jeremy Firestone
and James J Corbett, ‘Coastal and Port Environments: International Legal and
Policy Responses to Reduce Ballast Water Introductions of Potentially Invasive
Species’ (2005) 36 Ocean Development
and International Law 291, 292.
3International
Convention for the Control and Management of Ships' Ballast Water and Sediments, opened for signature 13 February 2004, ILM (not yet in force).
4 Secretariat of
the Convention on Biological Diversity, ‘Review of the Efficiency and Efficacy
of Existing Legal Instruments Applicable to Invasive Alien Species’ (2001) CBD
Technical Series no.2, 31.
5 Beeton RJS, Buckley Kristal I,
Jones Gary J, Morgan Denise, Reichelt Russell E, Trewin Dennis, ‘Independent Report to the Australian Government Minister
for the Environment and Heritage – State of the Environment Report’ (2006) Australian
State of the Environment Committee.
6 Sophie Riley,
‘Invasive Alien Species and the Protection of Biodiversity: the Role of
Quarantine Laws in Resolving Inadequacies in the International Legal Regime’
(2005) 17(3) Journal of Environmental Law 323,
331.
7 Convention on
Biological Diversity, opened for signature 5 June 1992, ILM, art 8(h) (entered into force 29
December 1993).
8United Nations
Convention on the Law of the Sea, opened for signature 10 December 1982, 1833 UNTS 3, art
196 (entered into force 16 November 1994).
9 Secretariat of
the Convention on Biological Diversity, ‘Review of the Efficiency and Efficacy
of Existing Legal Instruments Applicable to Invasive Alien Species’ (2001) CBD
Technical Series no.2, 7.
10 Ibid.
11 Secretariat of
the Convention on Biological Diversity, ‘Review of the Efficiency and Efficacy
of Existing Legal Instruments Applicable to Invasive Alien Species’ (2001) CBD
Technical Series no.2, 7.
12 Sophie Riley, ‘Invasive Alien Species and the Protection of Biodiversity:
the Role of Quarantine Laws in Resolving Inadequacies in the International
Legal Regime’ (2005) 17(3) Journal of Environmental Law 323,
336.
13 Ibid 335.
14 Secretariat of the Convention on Biological Diversity, ‘Review of the Efficiency
and Efficacy of Existing Legal Instruments Applicable to Invasive Alien
Species’ (2001) CBD Technical Series no.2,
9.
15 Sophie Riley,
‘Invasive Alien Species and the Protection of Biodiversity: the Role of
Quarantine Laws in Resolving Inadequacies in the International Legal Regime’
(2005) 17(3) Journal of Environmental Law 323,
333.
16 Secretariat of the Convention on Biological Diversity, ‘Review of the
Efficiency and Efficacy of Existing Legal Instruments Applicable to Invasive
Alien Species’ (2001) CBD Technical Series no.2,
14.
17 Ibid 15.
18 Ibid 16.
19 Sophie Riley, ‘Invasive Alien Species and the Protection of Biodiversity:
the Role of Quarantine Laws in Resolving Inadequacies in the International
Legal Regime’ (2005) 17(3) Journal of Environmental Law 323,
336.
20 Sophie Riley, ‘Invasive Alien Species and the Protection of Biodiversity:
the Role of Quarantine Laws in Resolving Inadequacies in the International
Legal Regime’ (2005) 17(3) Journal of Environmental Law 323,
334.
21 Secretariat of the Convention on Biological Diversity, ‘Review of the
Efficiency and Efficacy of Existing Legal Instruments Applicable to Invasive
Alien Species’ (2001) CBD Technical Series no.2,
18.
22 Australian
Quarantine and Inspection Service,
Australian Ballast Water Management Requirements
(2001).
23 Beeton RJS, Buckley Kristal
I, Jones Gary J, Morgan Denise, Reichelt Russell E, Trewin Dennis, ‘Independent Report to the Australian Government Minister
for the Environment and Heritage – State of the Environment Report’ (2006) Australian
State of the Environment Committee.
24 Jeremy Firestone and James J Corbett, ‘Coastal and Port Environments:
International Legal and Policy Responses to Reduce Ballast Water Introductions
of Potentially Invasive Species’ (2005) 36 Ocean Development and International Law
291, 293.
25 Michael Tsimplis, ‘Alien Species Stay Home: The International
Convention for the Control and Management of Ships’ Ballast Water and Sediments
2004’ (2005) 19(4) International Journal of Marine and
Coastal Law 411, 424.
26 Michael Tsimplis, ‘Alien Species Stay Home: The
International Convention for the Control and Management of Ships’ Ballast Water
and Sediments 2004’ (2005) 19(4) International
Journal of Marine and Coastal Law 411, 411.
27 Jeremy Firestone and James J Corbett, ‘Coastal and Port Environments:
International Legal and Policy Responses to Reduce Ballast Water Introductions
of Potentially Invasive Species’ (2005) 36 Ocean Development and International Law
291, 293.
28 Ibid 295.
29 Ibid.
30 Ibid 298.
31 Jeremy Firestone and James J Corbett, ‘Coastal and Port Environments:
International Legal and Policy Responses to Reduce Ballast Water Introductions
of Potentially Invasive Species’ (2005) 36 Ocean Development and International Law
291, 297.
32 Michael Tsimplis, ‘Alien Species Stay Home: The
International Convention for the Control and Management of Ships’ Ballast Water
and Sediments 2004’ (2005) 19(4) International
Journal of Marine and Coastal Law 411, 444.
33 Ibid 411.
34 See the IMO’s
2001 International Convention on the Control of Harmful Anti-Fouling Systems on
Ships (Anti-Fouling Convention).
35 Secretariat of the Convention on Biological Diversity, ‘Review of the
Efficiency and Efficacy of Existing Legal Instruments Applicable to Invasive
Alien Species’ (2001) CBD Technical Series no.2,
9.
36 Renae Leverenz,
‘Legislative Control of Invasive Species in Australia’ (2002) 13(1) Polemic
28, 31.
37 Jeremy
Firestone, ‘Dilemmas and Dimensions of Non-Indigenous Organisms and Pathogens
in the Marine Environment: A Sea Change’ (2006) 9 Journal
of International Wildlife Law and Policy 123, 127.
38 Sophie Riley, ‘Invasive Alien Species and the Protection of Biodiversity:
the Role of Quarantine Laws in Resolving Inadequacies in the International
Legal Regime’ (2005) 17(3) Journal of Environmental Law 323,
351.
39 Secretariat of
the Convention on Biological Diversity, ‘Review of the Efficiency and Efficacy
of Existing Legal Instruments Applicable to Invasive Alien Species’ (2001) CBD
Technical Series no.2, 24.
by Brian J. Brock
Photographs by Philip Hall, Fig 2c by Bob Baldock, sketches by Brian Brock
Three of my ancestors were early settlers; William
Archer Deacon, The Africaine
2/11/1836; Isabella Baird, The Navarino
1837; John Brock, The Lysander
1839. It is perhaps appropriate that I study early settlement at Glenelg. I am
concentration on the important ship foulers called Bryozoans. They are colonial
marine animals. Some are encrusting, but many look like small bushy algae.
Segments of coralline species are important fossils (River Murray cliffs,
Blanchetown, Overland Corner and Mount Gambier limestones;
Point Turton and Wardang Island flux quarries and sand deposits …..).
The first individual in the colony develops from a
planktonic larval stage that selects and settles in a spot to its liking. After
a short “resting” period, metamorphosis to a feeding zooid called an
ancestrula, takes place. The ancestrula may have characteristic spine counts
around the orifice from which ciliated tentacles of the polypide protrude. The
ancestrula buds in a particular way, producing other zooids of the same genetic
constitution, which bud in turn, developing the mature form of the colony.
Different species within a genus have different
spine counts and arrangements on the ancestrula. So it is possible to
distinguish between, for example, the different species of Bugula,
at the ancestrula stage. To make the counts, one has to collect ancestrulae and
examine them microscopically. The ancestrula and spines are fairly easily
damaged. If small bits of the substrate are collected from a zone in which a
particular bryozoan species is growing, in the hope of finding ancestrulae, the
substrate specimens must be handled carefully. They may be kept in fresh
seawater for immediate microscopic examination, or preserved (for example, in
80% methylated spirits, 10% deionised water, 10% glycerine). If fortunate
enough to possess an underwater microscope, development may be watched in
in-situ specimens. Live marine colonies will stay alive in fresh seawater in a
refrigerator, for a couple of days. Look at fresh live marine
and freshwater species.

Fig 1a: glass
plate araldited to grey cement-aggregate window-sill tile.

Fig 1b:
Two ground glass plates tied to a black plastic backing sheet & 6mm
acrylic.
A useful technique is to put tiles of some kind in
the water during the peak settlement period for a particular bryozoan species.
The main substrates I have used, are 8 x 10cm glass plates with a
diamond-belt-ground collecting surface, and cement aggregate window-sill tiles
(Brock, 1979a, 1985, 2006). Fig. 1a shows a glass plate araldited to a grey
cement-aggregate window-sill tile drilled for suspension horizontally 50cms
below the surface of the water beneath a floating marina-platform. The
collecting surfaces face downwards and bryozoan larvae and other propagules may
settle on the glass or the grey cement. Fig. 1b shows two of the ground glass
plates tied to a black plastic backing sheet and a 6mm acrylic sheet. The
acrylic is drilled for strong fishing-line across the corners of the glass
plates, and for wires that will attach the acrylic to a jetty-pile at a depth
of about 50cms below low Spring Tide Level. This depth of immersion has been
chosen because I am trying to collect ancestrulae of Tricellaria
porteri (originally described as Menipea
Porteri by MacGillivray in 1889). This
species is believed to settle below Low Spring Tide Level (Brock 2006).
Tricellaria occidentalis, the other species recently found at Glenelg (Brock
2006), settles above Low Spring Tide Level (Nielsen 1985; Anna Occhipinti
Ambrogi 1991, as Tricellaria inopinata
in Venice Lagoon).

Fig 1c:
Glass plates, one with black plastic backing, colonized on a Glenelg jetty-pile
between 5/12/06 and 8/1/07.
Fig. 1c shows two glass plates that were immersed at Glenelg Jetty on 5/12/06 and raised on 8/1/07. Only one of the glass plates has a black backing. The other was attached to the clear acrylic and was lighter in colour as a consequence. Bryozoans, algae and hydroids were amongst the wealth of marine organisms that settled on both plates. I showed them to Dr Bob Baldock while they were still alive. He took several photographs. One of the bryozoans was a pellucid bushy colony and is likely to be Tricellaria porteri (Fig. 2a). The other is the reddish bushy common jetty-pile and boat fouler called Bugula neritina. The tentacles of a B. neritina zooid are fully extended in Fig. 2b. I preserved the tile in 10% formalin-sea-water but left it for several months before trying to make ancestrula spine counts. By then, the wires had rusted and corroded and rust covered everything on the plates. It was not possible to make clear spine counts on the rusty colonies. Fig. 2c is an old photo, by Bob Baldock, of Tricellaria from a tile set below low tide level off Outer Harbour.
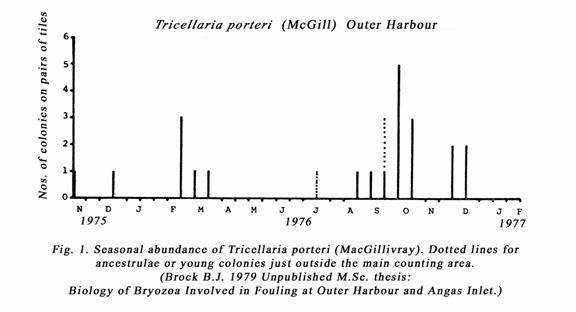
Histogram
of Brock 2006 (MLSSA Journal Number 16 Page 4)
As can be seen from the line histograms in Fig. 1 of
Brock 2006, the main settlement period for Tricellaria porteri occurred in Spring at Outer Harbour in 1976. I’ll put a new tile in at
Glenelg on 28/9/07 and take it out on 26/10/07. With a bit of luck, I might be
able to collect and make spine counts on early ancestrulae of Tricellaria
porteri, thereby settling the question of
its occurrence at Glenelg. (4/10/07. I was foiled by the waves on 28/9/07;
could not get near the proposed settlement site. Two tiles were set on 30/9/07.
They are due out on 28/10/07, waves willing!)

Fig 2a: A
pellucid bushy bryozoan,
possibly T.
porteri.

Fig 2b: Bugula
neritina with ciliated tentacles extended.

Fig 2c:
Dorsal view of Tricellaria
from a tile set below low tide level off Outer Harbour.
j.,
joint; l.a., lateral avicularium;
l.s., long spine; o., opesium;
r.c., root chamber.
THE DRIFT OF THINGS
(with respect to Roland Robinson, 1973; and Robert Frost,
1951).
Drift lines contain many treasures from the deep (or
shallows). Several lines can usually be seen, the highest indicating storm-tide
levels. If calm weather prevails drift lines indicate levels reached by Spring and Neap highs, or even the recent high high and low high of the 24 hour tidal rhythm. Seahorses,
pipefish, cuttlefish “bones”, puffer-fish or cow fish, shark and ray eggs,
pumice, plastic, light bulbs, gin bottles and other modern day artifacts are
common.

The Failsworth cap.
Recently, I retrieved a Failsworth
cap, a finely crafted light mooring rope and three golf balls from near the
mouth of the Patawalonga. Try as I might, I could not get all of the fine sharp
sand and foraminifera out of the hat. Beating it on a garden sieve over a large
sheet of newspaper yielded the forams I have drawn (Fig. 3). Fragments of bryozoans
and coralline algae, broken sponge spicules, sea urchin spines, ostracod tests,
and minute shells were also in the sand. Refer to Cann and Gostin (1985) for
some Port Gawler forams.
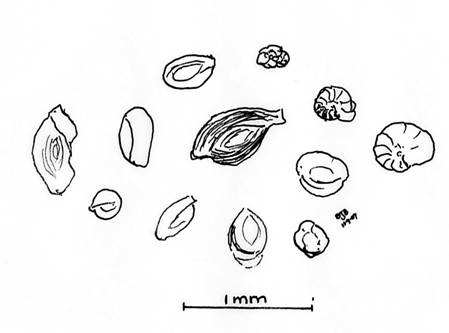
Fig.3:
Foraminifera from within the fabrics of a drift Failsworth cap.
W.J. Parr had a comprehensive article
on foraminifera in the July14th 1942 edition of the South Australian
Naturalist. The front cover of the edition has illustrations of 12 common St.
Vincent Gulf forams. He mentions Glenelg as a possible collecting site, and
raises the interesting idea of collecting and looking at living forams. Fresh
drift algae and marine flowering plants might be a good source of these. Shake
some healthy fresh drift with seawater in a wide-mouthed jar. Discard most, but
not all of the weed, and leave the jar near a
unilateral source of light. Forams should move to
the lighted side of the jar. I have not tried it, but it is high on my list of
priorities, as is making a little stage aquarium for my microscope so that
pseudopodia and movement of living forams may be observed (dark field illumination).
Following are some drawings of ancestrulae and
parts of young colonies of Tricellaria occidentalis from
tiles set on Glenelg jetty piles from 30/9/07 to 27/10/07. The tiles were
raised a day earlier than intended because inclement weather was predicted for
Sunday 28/10/07.
I have not found ancestrulae with only 6 spines, so I
have not been able to prove that Tricellaria
porteri still occurs in our waters. Most
of the Tricellaria occidentalis colonies
on the piles are above Low Spring Tide level, so I was a bit surprised to find
it settling on tiles set lower than that.
Watersipora and at least two
other bryozoan species settled on the pitted (with a hot wire) acrylic.
Small colonies were transferred to fresh seawater held in
a Perspex ring stuck to a microscope slide with Bostik Super Glue. A fine camel
hair brush, was used for the transfer. Most of the hairs
were cut off the brush, so it was really fine and very small colonies were
picked up without damage. The colonies were freed from the tiles using the tip
of a Utility Cutter blade.
Spine
counts were made at 100x using a compound microscope. Drawings were made using
a camera lucida kind of drawing attachment on an Olympus ECTr3 microscope. The
Perspex mini-aquarium, did not have a coverslip on it.
The little colonies being well submerged, there was no distortion of the image.
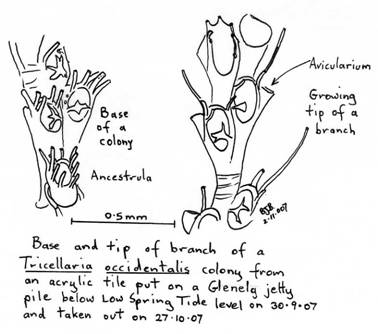
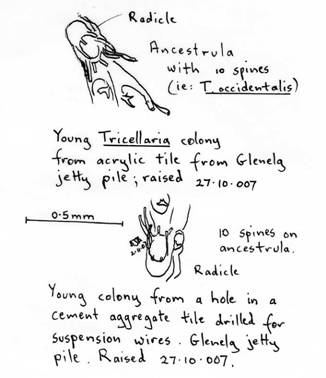
GREENHOUSE
GASES WITHOUT COWS
Shallow lower intertidal pools have developed
just east of the southern breakwater near the mouth of the Patawalonga due to
wave wash. I noticed gas bubbling up in these pools and from other black sand
areas between and just south of the breakwaters. Hydrogen peroxide turned the
black sand lighter and the water near the bubbling sand milky. The smell of
hydrogen sulphide (rotten egg gas or sewer gas) was quite noticeable, as it is
in other areas where a lot of rotting algae and marine flowering plants
accumulate. The milkiness of the water was due to the splitting of the hydrogen
sulphide by the hydrogen peroxide, releasing colloidal sulphur.
The
smell did not seem bad enough for all of the gas to be hydrogen sulphide
(remembering several years of the stench near the Kipp’s Apparatus in
qualitative analysis Chemistry laboratories).
A
second test solved the mystery. I went to the pools with a jam-tin,
preserving-jar lid, and a box of matches. Finding a good stream of bubbles in a
still pool at Low Spring Tide, I dug out the spot sufficiently to collect a tin
full of gas by displacement of sea-water. The tin of gas, with lid on, was
taken to a spot on firm sand nearby. I dried my hands, slid the lid aside, and
put a lighted match to the gas. It burnt down quietly with a pale blue flame (a
little colour). There was no smell of burning sulphur, or sulphur deposit. I
concluded that the gas was mainly methane. The gases are produced by microbial
action on decaying organic matter, mainly algae and marine flowering plants,
but also associated animals (Eltringham, 1971, pp.10 & 11; & 190 &
191).
I
have seen gas bubbling up from black mud near the mouth of the Torrens, but
have not tested it. I have done the peroxide test for hydrogen sulphide in
several places. Lead acetate paper could also be used (turns black).
My
eloquence about methane bubbling up from black sand at Glenelg, stirred friends
to pass on information about methane clathrate, or burning ice. Go to Wikipedia
for details and many references. Enough methane is trapped in the ice lattice
under certain conditions, for the ice to burn when heated (the methane burns;
the ice melts).
The
production of methane and hydrogen sulphide by bacterial activity in beach
muddy sands, and in the gut of cows and humans etc. is natural. Nature at work. Let it be.
BEEN THERE DONE THAT (or have we?).
I continue to be surprised at what I find or observe on “the same old beach”. Storms often wash up something new. A recent find south of the southern breakwater was the rock shell Cleidothaerus albidus Lamarck 1819. I have not found it before, but there were three specimens cast up, one tightly closed. The right valve adheres tightly to a rock. Without the flat left valve, it looks like a little pearly armchair. My eyes must have passed over Cotton’s illustration dozens of times without registering it (Cotton 1976 Plate II no.21). I have not yet seen an attached specimen. Time I had another snorkel!!

Right valve: mother of pearl armchair.
Cleidothaerus albidus

Right valve: showing the flat surface that adheres to
rock.
Cleidothaerus albidus
In the 70’s, I found a razor shell containing
octopus eggs, just north of the Patawalonga outlet. Nearby was a moribund
octopus (battered and exhausted). I put the octopus and what I presumed were
its eggs, in a bucket of fresh seawater. The octopus slowly began pumping water
and showing more signs of life. Later, in a seawater aquarium in our Currie
Street laboratory, the octopus became even more active, found its eggs and sat
over them, forming a protective chamber with its body, through which it
continuously pumped water. It brooded and cared for its eggs, tending them with
the tips of its tentacles, until they hatched, several weeks later. It then
died. After a recent blow, I found another razor-fish shell containing eggs on
17/8/07, in the same spot. I did not collect them then, but on Sunday thought
maybe I could do an egg count. On Monday 19/8/07, I picked up the shell and
washed it in the sea. To my surprise, there were two batches of eggs in the
shell, one on each valve. One batch had been completely covered with sand as
the shell was cast high up on the beach. The covered eggs looked to be in
better condition than those that had been exposed to heat and air flow under
their little “carport”. There were about a hundred eggs in each batch, each egg
being attached to the shell by a slender stalk. The eggs looked like pendulous
teardrop pearls. To quote from an illustrated poem – article that appeared in
the December 1979 Red Gum magazine (Brock, 1979b):
Octopus eggs in old
razor shells
are brooded and guarded –
life for many
for one discarded.
I did not put the 19/8/07 eggs in an
aquarium. The little octopi within them, were well
developed, and it is likely that some would have hatched in a well aerated
aquarium. Then would have come the problem of feeding
them. The best solution would perhaps be to put them back in deeper clear water.
(A tasty dish for a hungry fish without mother’s
protection?).

Pinna
bicolor
Octopus
eggs

Just
hatched octopus
STICK-ON-THE-PILES OR BREAKWATERS.
How do these stay at homes feed and reproduce? (See: Heide & Heide 1972 for Galeolaria).
Some answers can be found by direct observation of jetty-pile or rock pool
organisms using a mask, or underwater viewer. Specimens dislodged by storms may
revive in a shallow pool or container of seawater. The development of embryos
of some marine invertebrates can be followed in simple aquaria. The chapter on
Molluscan egg masses in Part II of Marine Invertebrates of Southern Australia
assists in identifying some of the egg masses found in drift lines or where
laid (Smith, Black & Shepherd, 1989). What eats the mussels, periwinkles,
warreners, sea-urchins, pheasant shells, seasquirts, bryozoans and barnacles?
What marine creatures can lift abalone or limpets from their rocks? Slowly you
can find out.
The National Geographic Magazine has some
spectacular photographs of marine invertebrates eg: Nov. 1973 on barnacles
(Starbird & Sisson); Feb. 2007 Hawaii’s unearthly worms (Holland & Murawski). See also: Dakin-Bennett (1987);
Take a x10 hand lens
to fresh drift algae and marine flowering plants in a shallow dish of fresh
seawater. Spirorbids and other segmented worms,
bryozoans, hydroids, foraminifera, crustaceans, and delicate epiphytic algae
might be seen. How did they get there? What do they eat?
INDIGENOUS AND EXOTIC COASTAL PLANTS.
The pocket of sand high up the beach in the southern
angle of the breakwater adjacent to the Patawalonga outlet, and the fringing
gardens, support some of the original coastal-dune flora, and several
introduced salt-fallout-tolerant species. It is not the place for Norfolk
Island Pines, but Metrosideros
is happy enough. Surfactants in runoff and sewage effluent,
make it easier for salt to penetrate and damage the leaves of coastal plants.
Sea Rocket (Cakile)
and coastal spinifex (Spinifex sericeus)
are fairly tolerant of sand blasting and salt fall-out. Nitraria
schoberi, Atriplex
cinerea, Leucophyta
brownii, Isolepis
nodosus, Olearia
axillaris and Carpobrotus
rossii are also fairly tolerant. More
sensitive coastal species could be planted in the lee of the tall flats. The
buildings act rather like dunes, shielding plants behind them from much of the
salt and fierce winds. More use could be made of our attractive flowering and
brightly fruited coastal natives. Look at Bagust and
Tout-Smith (2005), Dashorst and Jessop (1990), and Kraehenbuehl (1996), for
excellent illustrations. Neville Forde presents the results of a decade of scat
analysis and careful field observation of feeding of birds on coastal and
inland plants (Forde, 1986). Don’t forget the woody fruited species like Allocasuarina
verticillata, Banksia
marginata, Callitris
gracilis and the two common coastal Melaleuca
species. Parrots can handle these. I even saw silver gulls gorging on seeds of
an introduced Pinus
species at Coobowie during one extremely hot spell. The dry heat had caused most
of the mature pine cones to split open. Many wattle species have prominent
arils or funicles popular with coastal birds. (Whibley &
Symon, 1992). If fortunate enough to have mistletoes on woody coastal
species, do not cut them out. They provide fruits, nectar, and associated
insects for honeyeaters and other birds (Reid, 1986; Forde, 1986).
In these parching times, it is salient to
remember that no-one watered our diverse and colourful dune flora.
AUSTRALIA’S ENTAIL
“What a priceless entail we have in Australia – our
natural indigenous plants.” A.O. Barrett (1937). For the purpose of this
exercise, I would say our coastal plants and animals. How much can we hand on?
Beware lest carparks, marinas and highrise completely alienate the Coastal
Commons. Some introduced species need controlling but I am not against all. The
pine-cone confetti beneath Pinus
species, attests to their value as food for parrots. Rabbits, rats, cats and
foxes do have to be controlled if we wish to preserve coastal native plants,
birds and little animals. Our native species entail, is a source of the best
ecotypes for the local area. I have been privileged to wade and wander and swim
with some for decades, but I have only dipped the brush in the paint. Remember
to look at the mosses and lichens.
REFERENCES
Bagust P. & Tout-Smith L. (2005) The
Native Plants of Adelaide (Dept. for Environment & Heritage, Adelaide).
Barrett A.O. (1937) Australia’s Entail
(Robertson and Mullens Ltd., Melb.).
Brock B.J. (1979a) Biology of Bryozoa Involved in
Fouling at Outer Harbour & Angas Inlet. Unpublished M.Sc.
thesis Zoology Dept. University of Adelaide.
Brock B.J. (1979b) Eggs on the marine scene. Red Gum 3 (4) pp 10
& 11.
Brock B.J. (1985) South Australian fouling
bryozoans. In:
Nielsen C. & Larwood G.P. (Eds.): Bryozoa: Ordovician To
Recent (Olsen & Olsen) pp. 45-49.
Brock B.J. (2006) Evidence for the occurrence
of three fouling bryozoan species ….. in Adelaide
waters. MLSSA Journal No. 16 pp. 4-8.
Cann J.H. & Gostin V.A. (1985) Coastal
sedimentary facies & foraminiferal
biofacies of the St Kilda Formation at Port Gawler,
South Australia. Trans. R. Soc. S.Aust. 109(4)
pp. 121-142.
Coleman N. (1977) A Field Guide to Australian
Marine Life (Rigby Ltd.).
Cotton B.C. (1976) South Australian Shells
(S.A. Museum).
Dakin W.J. & Bennett I. (1987)
Australian Seashores (Angus and Robertson).
Dashorst G.R.M. & Jessop J.P. (1990) Plants of
the Adelaide Plains & Hills (Botanic Gardens of Adelaide).
Eltringham S.K. (1971) Life in Mud & Sand
UNIBOOKS
Forde N. (1986) Relationships between
birds & fruits in Temperate Australia. In:
Ford H.A. & Paton D.C. The Dynamic Partnership (S.A.
Govt. Printer) pp. 42-58.
Frost R. (1951) Complete Poems. Reluctance (3rd
line of last stanza on p 50).
Heide C. & Heide E.K. (1972)
Fertilization in tube worms (Galeolaria caespitosa). SASTA journal no. 723 pp. 63 & 64.
Holland J.S. & Murawski
D.A. (2007) Hawaii’s unearthly worms. National Geographic 211
(2) pp. 118 – 131.
Kraehenbuehl D.N. (1996) Pre-European
Vegetation of Adelaide: A Survey from the Gawler River to Hallett Cove. (Dept. of Housing & Urban Development. S.A.)
MacGillivray P.H. (1889) On some S.A. Polyzoa
Trans. R. Soc. S.Aust. 12
pp. 24-30 & Plate II.
Nielsen C. (1985) Ovicell
formation in Tegella
& four cellularioids (Bryozoa, Cheilostomata). In:
Nielsen C. & Larwood G.P. (Eds) Bryozoa:
Ordovician to Recent (Olsen & Olsen) pp. 213-220
Occhipinti Ambrogi A. (1991) The spread of Tricellaria
inopinata into the lagoon of Venice: an
ecological hypothesis. In:
Bigey F.P. (Ed.) Bryozoaires
Actuels et Fossiles: Bryozoa Living & Fossil. Bull. Soc. Sci. Nat. Ouest Fr., Mém. HS1 pp. 299-308.
Parr W.J. (1942) Foraminifera. The South Australian Naturalist.
21 (3) pp. 1-9.
Reid N. (1986) Pollination and seed
dispersal of mistletoes (Loranthaceae) by birds in
southern Australia. In:
Ford H.A. & Paton D.C. The Dynamic Partnership (S.A.
Govt. Printer) pp. 64-84.
Robinson R. (1973) The
Drift of Things. (Macmillan)
Smith B.J., Black J.H. & Shepherd S.A.
(1989) Molluscan egg masses & capsules. In:
Shepherd S.A. & Thomas I.M. (Eds.) Marine Invertebrates of Southern
Australia Part II (S.A. Govt. Printer) pp. 841-891.
Starbird E.A. & Sisson R.F. (1973)
Friendless squatters of the sea. National
Geographic 144(5)
pp. 623-633.
Whelan H. & Deacon K. (1993) Explosion of
life the nights the reef went off. Australian Geographic no. 32 pp. 32-55.
Whibley D.J.E. & Symon D.E. (1992)
Acacias of South Australia. (S.A. Govt. Printer)
Wikipedia. http://en.wikipedia.org/wiki/Methane_clathrate.
The Flora & Fauna Of Piccaninnie Ponds And
Ewens Ponds (Including Eight Mile Creek) - Part 2
by Steve
Reynolds
My
article “The Flora & Fauna Of Piccaninnie Ponds And
Ewens Ponds (Including Eight Mile Creek)” in our 2006 Journal was essentially
the continuation of one that was featured in the September 2006 issue of Dive
Log Australasia (“MLSSA’s 2006 Mount Gambier Trip”). That article described our
trip in detail whereas the Journal article discussed the flora and fauna of the
ponds in detail.
The
article which featured in the Journal was, unfortunately, not the final version
that was intended. The final draft was apparently not received by
the Editor so one of the early drafts was published in error.
I became concerned that some information was missing whilst I was re-reading my
own work in the Journal. A comparison of the Journal article with my final
draft revealed that some of the intended information was missing. It is now my
aim to rectify the error.
Page 49
of the Journal featured a list of “the common reeds and bulrush that dominate
the area surrounding Ewens Ponds” (two items). The article then went on to say
“Tea-tree thickets consisting of Leptospermum pubescens and Scented paperbark, Melaleuca squarrosa are scattered
amongst the reeds and bullrush. These vegetation
associations (in the upper reaches of the ponds) have root systems which
stabilize the banks and prevent contamination by surface runoff.”
A
sentence explaining that “The Biology of Ewens and Piccaninnie Ponds, South
Australia” by Dr. Neil Hallam
discusses the vegetation of the
ponds in detail, including Leptospermum
and Melaleuca
species” was missing after this. It
should have featured just before the reference to my list of the vegetation
(plant and algae species) known to occur in Ewens Ponds.
The actual list of the vegetation known to occur in Ewens
Ponds was featured on page 50 of the Journal. This list, however, was
incomplete. It should have listed 20 species including the following at the
bottom of the page: -

Common Name
|
Scientific Name
|
Family
|
Common Duckweed
|
Lemna minor
|
Lemnaceae
|
Duckweed
|
Lemna triscula
|
Lemnaceae
|
Moss
|
Cratoneuropis relaxa
|
|
Speedwell
|
Veronica catenata
|
Scrophulariaceae
|
Water Milfoil
|
Myriophyllum species
|
Haloragaceae
|
Sea Tassel
|
Ruppia maritoma
|
Potamogetonaceae
|
Some
paragraphs in the Journal article were placed in positions different to the
intended final draft version. This does not seem, however, to spoil the
message. Part of the intended text was omitted from the Journal article though,
including one of my lists. This text is now reproduced below: -
“The
Biology of Ewens and Piccaninnie Ponds, South Australia” by Dr. Neil Hallam says that, “The dominant species before drainage was
watercress, Nasturtium officinale,
occurring there in great masses from deep in the (Ewens) ponds to the surface.
At the present time (1983-5) it is mainly restricted to Eight Mile Creek
between the ponds and along the edges of Eight Mile Creek as it flows from the
third pond to the sea”.
“The
Biology of Ewens and Piccaninnie Ponds, South Australia” also says that
pondweed “Potamogeton
pectinatus is only found
now (1983-5) in the lower reaches of the creek and at Piccaninnie Ponds. It
also says that “Myriophyllum,
once recorded for Ewens Ponds by Eardly*, is now
found only at Piccaninnie Ponds. Myriophyllum
elatinoides has not been
seen in Ewens Ponds or Eight Mile Creek over the last five years (to 1983-5),
although it was recorded by her in 1943”.
Constance
Eardly carried out an ecological survey of Ewens
Ponds in 1943. It seems that her results were published in the Proceedings of
the Royal Society of South Australia that same year.
“The
Biology of Ewens and Piccaninnie Ponds, South Australia” repeats the comment
that “Potamogeton pectinatus occurs only in
the lower reaches of Eight Mile Creek (beyond the third pond). It also says the
same for the Sea Tassel, Ruppia
maritoma.
The
vegetation of Eight Mile Creek is said to be “dominated by clumps of green Nasturtium*
and red purple Veronica catenata.
* The
watercress Nasturtium officinale
(also called Rorippa officinalis or Rorippa nasturtium-aquaticum or Radicula nasturtium-aquaticum).
The
freshwater red alga, Batrachospermum
species is “locally abundant” but it is often classified as rare. It is said to
be present within the small cave (overhang) at the bottom of the third pond and
also beneath the landing of the first pond at Ewens Ponds.
The
channels between the ponds at Ewens Ponds are said to be dominated by the
watercress Rorippa
nasturtium-aquaticum,
the Lesser Water parsnip,
Berula erecta
(or Sium latifolium)
and the common spike-rush (Eleocharis acuta).
And . . .
According
to Dr. Neil Hallam, the tea tree thicket Leptospermum
lanigerum is said to
surround Piccaninnie Ponds “and bog plants such as Typha
(bullrush), Cladium
and Eleocharis
(sedges).”
This next part of my list covers
some of the vegetation (plant and algae species) known to occur in Piccaninnie
Ponds: -

Common Name
|
Scientific Name
|
Family
|
River buttercup
|
Ranunculus amphitrichus
|
Ranunculaceae
|
Water ribbons
|
Triglochin procerum (or procera?)
|
Juncaginaceae
|
Shield pennywort
|
Hydrocotyle verticillata
|
Apiaceae
(formerly Umbelliferae)
|
Duckweed
|
Lemna triscula
|
Lemnaceae
|
Watercress
|
Rorippa
nasturtium-aquaticum (also called Rorippa officinalis or Nasturtium officinale or Radicula nasturtium-aquaticum) |
Brassicaceae (Cruciferae)
|
Blue-green bacteria/alga
|
|
Division:
Cyanobacteria
|
Moss
|
Distichophyllum microcarpum
|
|
Water Milfoil
|
Myriophyllum propinquum
|
Haloragaceae
|
Saw sedges
|
Gahnia spp
|
Cyperaceae
|
Rushes
|
Juncus spp
|
Juncaceae
|
Rushes
|
Scirpus spp
|
Cyperaceae
|
Total: 11
|
|
|
According
to Dr. Neil Hallam, “Large clumps of Triglochin procera also dominate
the pond edges. Some of the other plants that grow at Ewens Ponds such as Ranunculus, Nasturtium and Hydrocotyle
do not grow as robust at Piccaninnie Ponds presumably because of the higher
salinity. The swamp system surrounding the ponds is dominated by tussock
species such as saw sedges (Gahnia
spp) and rushes (Juncus
and Scirpus spp).”
(As
indicated in the previous Table, Gahnia
spp and Scirpus
spp belong to the Cyperaceae family and Juncus
spp belong to the Juncaceae family.)
Dr Hallam also says that Water Milfoil, Myriophyllum propinquum, does not
occur at Ewens Ponds. In Piccaninnie Ponds, however, it “grows as a submerged
aquatic at the edges of the chasm, usually with filamentous algae entangled in
it”.
He also
says that, “The aquatic moss Distichophyllum
microcarpum is another
species not present at Ewens Ponds and it grows down the walls of the chasm to
depths of 16metres”.
He says
that, “The only other plants growing down into the chasm are blue-green algae,
these purple tufts contrasting with the bright green 2-3 cm high clumps of Distichophyllum on
the limestone ledges . . .”.
He also
says that, “Large clumps of Lemna
triscula . . . can be
seen on the ledges within the chasm and in the swamp surrounding. This species
is quite rare at Ewens Ponds but grows well in the harder, more saline waters
of Piccaninnie Ponds”.
I trust that the above clarifies any
discrepancy in the original Journal article.